Electrolysis of sodium chloride is an important process to manufacture many bulk chemicals of commercial utility, like chlorine, sodium hydroxide etc. Sodium chloride is electrolysed either in a molten state or in aqueous solutions. Besides, electrolysis is done in the presence of additional salts to aid the redox reactions.
Download Complete Chapter Notes of Electrochemistry
Download Now
Electrolysis of Molten Sodium Chloride
Electrolysis involves the movement of ions to the electrode. Solid-state does not allow the movement of ions and is unsuitable for electrolysis. When melted at high temperatures, sodium chloride separates into sodium and chloride ions so that electrolysis can take place to form sodium atoms and chlorine gas.
Read More in Detail: Electrolysis

NaCl → Na +(l) + Cl–(l)
At cathode: reduction of 2Na+(l) + e– → Na(l)
At anode: oxidation of 2Cl–(l) → Cl2(g) + 2e–
Net reaction is written as: 2Na +(l) + 2Cl–(l) → 2Na(l) + Cl2(g)
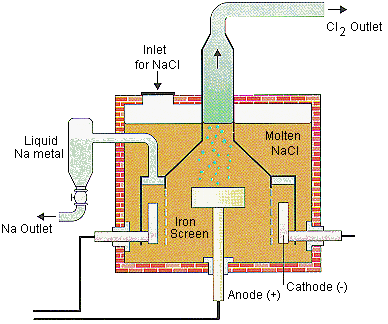
Down’s Process: Sodium chloride melts at a very high temperature of 801°C. Addition of anhydride calcium chloride in the ratio of CaCl2: NaCl = 3:2 reduces the melting point to 580°C. Electrolysis is done with an iron cathode and graphite anode, and iron gauze preventing the mixing of products, chlorine and sodium. The products of molten sodium chloride are sodium metal and chlorine gas.
Electrolysis of Aqueous Sodium Chloride
Sodium chloride is dissociated and exists as sodium and chloride ions in aqueous solution. Electrolysis of sodium chloride is easier in aqueous solution. But the water itself can undergo reduction and oxidation reactions at different potentials. So, the substance that is oxidised or reduced is not sodium and chloride ions alone, but it may involve the water molecule also.
Also Read: Water Electrolysis
Two competing reactions are possible at both the cathode and anode. At cathode: reduction reaction: at pH = 7.
Water can be reduced to hydrogen gas or sodium ions reduced to sodium metal.
2H2O(l) + 2e– → H2(g) + 2OH– E° = -1.0 V
Na+(l) + e– → Na(l) E° = -2.71V
At anode: oxidation reaction: at pH = 7. Water can be oxidised to oxygen or chloride ion oxidised to chlorine molecules.
2H2O → O2(g) + 4H+ + 4e– E° =-1.42 V
2Cl– → Cl2 + 2e– E =- 1.36V
Hence, the product of electrolysis of aqueous sodium chloride can be anything between,
i) Sodium metal or hydrogen gas at the cathode
ii) Chlorine or oxygen gas at the anode
With a side product of sodium hydroxide resulting from the reaction of sodium and water, the product of electrolysis depends on the concentration of sodium chloride aqueous solution.
a) Very Dilute Aqueous Sodium Chloride Solution
Water has very low conductivity, and the small amount of ionic sodium chloride helps the ionic conductivity of the solution. In small concentrations, the electrolysis of water becomes more predominant yielding hydrogen at the cathode and oxygen at the anode.
At cathode: 2H2O + 2e– → H2(g) + 2OH– E° = -1.0 V
At anode: 2H2O → O2(g) + 4H+ + 4e– E° = +1.4 V
The net reaction of electrolysis of very dilute aqueous sodium chloride is given as,
2H2O → H2(g) + 2OH– + O2(g) E° = – 2.4 V
b) High Concentration of Sodium Chloride
At cathode: reduction reaction: at pH =7
2H2O(l) + 2e– → H2(g) + 2OH– E° = -1.0 V
Na+(l) + e– → Na(l) E° = -2.71V
The reduction (over) potential of water being more positive than the reduction of sodium ions, water is decomposed to liberate hydrogen at the cathode.
At anode: oxidation reaction: at pH =7
2H2O → O2(g) + 4H+ + 4e– E° =-1.4 V
2Cl– → Cl2 + 2e– E =- 1.36V
The thermo-dynamical reduction potential of water and chloride is +0.82 V and 0.1.36 V, respectively. Oxidation of water being more positive is more feasible, so the evolution of oxygen gas should happen at the anode. But, the evolution of oxygen from water has an overvoltage of -0.6V, making the voltage for the oxidation of water as -1.4V. Chloride oxidation is more positive than the net voltage of water oxidation. So, chloride is, oxidised to chlorine at the anode.
The net reaction taking place is given as follows:
At cathode – 2H2O(l) + 2e– → H2(g) + 2OH–
At anode – 2Cl– → Cl2 + 2e–
The overall reaction is as follows:
2H2O(l) + 2Cl– → H2(g) + Cl2 + 2OH–
Or \
2H2O(l) + 2NaCl → H2(g) + Cl2 + 2NaOH
At high salt concentration, hydrogen and chlorine is the product of electrolysis with sodium hydroxide as a by-product.
In the Castner-Kellner process, brine (aqueous sodium chloride) is electrolysed in a cell having two compartments. The graphite anode is in the side compartments, and the iron cathode is in the central compartment. Mercury is at the bottom and separates the two compartments and acts as a conduit for carrying sodium formed at the end compartments to the central compartment.
Brine, added to the end compartment, is electrolysed to sodium and chlorine. While chlorine escapes at the top of the cell, sodium forms sodium amalgam with mercury at the bottom. Sodium transferred to the central compartment reacts with water to form sodium hydroxide and hydrogen.
The products of electrolysis of concentrated aqueous sodium chloride are sodium hydroxide, hydrogen gas and chlorine gas.
Mercury used in the Caster-Kellner process contaminates the products and is an environmental hazard due to sublimation. Mercury being carcinogenic, is eased out for the electrolysis of aqueous sodium chloride.
Presently, all the Chlor-alkali industries, producing chlorine and sodium hydroxide, use a semipermeable membrane ‘Nafion’ to separate the anode and cathode compartments.

Nafion is a sulfonated ionic tetra fluoro-ethylene copolymer. The membrane allows the sodium ion to flow across the membrane into the cathode cell. Additional reinforcing with other semipermeable membranes prevents any back mixing of incoming chloride and hydroxide ions across Nafion.
c) Electrolysis of Aqueous Sodium Chloride in Intermediate Concentration
At cathode: The reduction of water to hydrogen is more feasible than sodium hydrogen. In all aqueous solutions, the reduction of water to hydrogen takes place at the cathode compared to the reduction of sodium ions.
At anode: The oxidation potential of water and chloride ions are almost the same (-1.4 V and 1.36 V, respectively).
At higher concentrations of sodium chloride, chloride oxidation is favoured kinetically, while at low concentrations, oxidation of water to hydrogen is favoured kinetically. At intermediate concentrations, both water and chloride ions are oxidised. So, both oxygen and chlorine gases evolve at the anode simultaneously.
At cathode: reduction reaction: at pH =7, 2H2O(l) + 2e– → H2(g) + 2OH–
At anode: oxidation reaction: at pH =7 2H2O → O2(g) + 4H+ + 4e–
2Cl– → Cl2 + 2e–
Reduction reaction x 3 6H2O(l) + 6e– → 3H2(g) + 6OH–
So, the net reaction is,
4H2O(l) + 2Cl– → 3H2(g) + 2OH– + Cl2 + O2
Or
4H2O(l) + 2NaCl → 3H2(g) + 2NaOH + Cl2 + O2
Sodium chloride electrolysis in an aqueous solution yields different products depending on the relative concentration of sodium chloride and water. The electrolysis is thermodynamically controlled at very low and high concentrations of sodium chloride and kinetically controlled at inter-mediatory concentrations.
Also, Check out:

Comments