What Is Electrolytic Refining?
Electrolytic refining is a process of refining a metal (mainly copper) by the process of electrolysis. As far as the mechanism of the process is concerned, during electrolysis, a large chunk or slab of impure metal is used as the anode, with a thin strip of pure metal as the cathode. In this setup, an electrolyte (metal salt aqueous solution), depending on the metal, is often used.
The clean or pure metal is formed at the cathode when the electrical current of a sufficient voltage is applied by dissolving impure metal at the anode. Electrolytic refining is also sometimes referred to as electrorefining.
Also Read: Electrolysis
Electrolytic Refining of Metals
The below-given table outlines the methods used to refine five metals. It is necessary to choose the electrolyte and other conditions so that both anodic dissolution and metal deposition proceed with high efficiency while none of the impurity metals can move from the anode to the cathode. Clearly, there must be no passivation of the anode, and the aim is to produce a deposit at the cathode of good quality, sometimes extremely crystalline. Additives are applied to the electrolyte when necessary to impose the right behaviour on both electrodes.
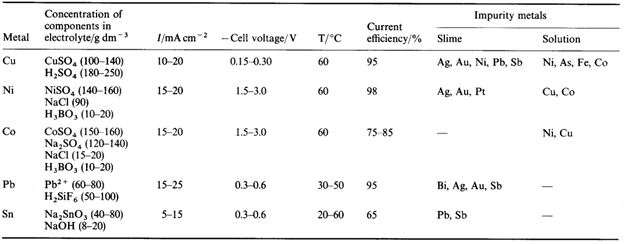
Electrolytic Refining of Copper
Here, we will take an example of electrolytic copper refining to understand the process more clearly. Copper is usually mined from coal, known as blister copper. It is about 98 to 99 per cent pure. However, the electro-refining process can easily make it 99.95% pure, which makes it a good product to be used in electrical components.
A block of impure copper is taken as an anode or positive electrode. Copper sulfate, which is acidified with sulphuric acid, is used as a graphite-coated electrolyte, along with pure copper tubes, as a cathode or negative electrode. In this phase of electrolysis, copper sulfate divides into a positive ion of copper (Cu++) and a negative ion of sulfate (SO4—). The positive copper ion (Cu++) or cations travel towards the negative electrode made of pure copper, where it absorbs the electrons from the cathode. Cu atom is deposited on the cathode’s graphite layer.
The cathode is coated with graphite in the process of electrolytic metal processing or merely electro-grinding so that the concentrated material can be easily removed. This is one of the most growing electrolysis procedures.

However, the anode’s metallic impurities are also mixed with SO4, forming metallic sulfate in the electrolyte solution and dissolving. Impurities such as silver and gold that are not produced by a solution of sulphuric acid-copper sulfate settle down as the anode sludge or dust.
Also Read: Water Electrolysis
The deposited copper is then separated from the cathode and anode and is replaced by a new block of raw copper at regular intervals for electrolytic copper refining.
Properties and Uses of Copper
Copper is a major commercial metal. Some properties of copper are listed below:
- It has high electrical and thermal conductivity.
- Easily moulded or ease for casting, extrusion, and rolling.
- Low corrosion level.
- It has excellent alloy characteristics.
- It has high aesthetic appeal and low human toxicity.
In modern times, the main use of copper is in the form of an electrical conductor. Copper has a very high per unit volume electrical conductivity. It can be easily drawn through strings, whether single or multi-filament, which can be conveniently and regularly twisted without undue toughness of operation. Copper wire is readily tinned, has superior soldering properties and is resistant to corrosion at points of contact.
Electrolytic Refining of Minerals
The electrolytic refining processes of certain major minerals are discussed below:
Gold
In the process of electrolytic gold refining, hydrochloric acid is used as an electrolyte. The cathode is made of a thin sheet of gold, and the anode is made of a gold alloy. When ion transfer takes place, pure gold with a higher purity rate is transferred to the cathode. This method is also called as the cycle of Wohlwill.
Silver
Similarly, the anode is made of crude silver, and the cathode is made of more refined silver. The electrolytic process is the same as discussed above. The only exception or difference is that the nitric acid bath is used where the silver anodes get dissolved. Thus, pure silver of about 99.9 per cent is obtained.
Refining Process Against Impurities
Grinding and refining play a crucial role throughout metallurgy. Every metal obtained from its ore is typically unclean in nature. Refining is a way to remove impurities and produce high-purity metals. Impurities are extracted by different methods from raw metal depending on material properties and impurities. Some different techniques apart from electrolytic refining or electrolysis that are used in crude metal purification include,
- Distillation
- Liquation
- Zone refining
- Vapour phase refining
- Chromatographic methods
Electrolytic refining is a much more common process than electro-winning, and such plants exist on a scale ranging from 1000 to 100,000 tons per year throughout the world. These are typically part of a larger project to extract raw metals from both waste and main ores and retrieve them.
Also Read: Electrochemistry
The system must, therefore, be designed to handle a metal feed of variable quality and result in a concentrate of all the materials present in a shape that can be further processed. Electrorefining also offers a very high metal purity.
Using a molten salt or non-aqueous electrolyte, electrorefining methods are used and are indeed the focus of further research. This is due to the possibilities they offer to increase current densities and refining through lower oxidation states that are not stable in water (For example, copper refining via Cu+ would nearly halve the energy requirement).

Comments