Aryl halides, better known as haloarenes is an important topic in organic chemistry included in the JEE syllabus. Questions based on their reactions and methods of preparation are asked almost every year in JEE.
Important Questions For Haloarenes
Some FAQs related to aryl halides are:
Question: What are haloarenes?
Answer: Haloarenes or aryl halides are chemical compounds containing arenes, where one or more hydrogen atoms bonded to an aromatic ring are replaced with halogens. They are generally denoted by “Ar-X” where ‘X’ denotes the halogen atom attached and ‘Ar’ stands for an aryl group.
Questions: What are the general methods of preparation of haloarenes?
Answer: Haloarenes can be prepared from other organic compounds by numerous methods. Some of them are underlined below:
- Electrophilic substitution reaction: Aryl halides can be prepared by electrophilic aromatic substitution of arenes with halogens in the presence of a Lewis acid.

Figure: electrophilic substitution reaction
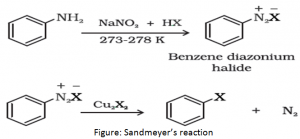
- Sandmeyer’s reaction: Aryl halides can also be prepared by mixing the solution of freshly prepared diazonium salt from the primary aromatic amine with cuprous chloride or cuprous bromide.
Question: What are some of the important reactions of haloarenes?
Answer: The C-X bond in haloarenes is quite strong in comparison to C-X bond in haloalkanes as the halogen atoms in haloarenes are bonded to sp2hybridized carbon atoms having more of s character in comparison to haloalkanes where it is bonded to sp3 hybridised carbon atoms. Therefore it is not reactive towards nucleophilic reactions. Some important reactions of haloarenes are underlined below:
- Friedel Crafts reaction: Haloarenes undergo electrophilic aromatic substitution reaction when treated with an alkyl halide in the presence of a Lewis acid.
- Wurtz-Fittig reaction: When a mixture of alkyl halide and aryl halide is treated with sodium in dry ether alkylarene is formed. This reaction is known as Wurtz-Fittig reaction.
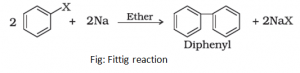
- Fittig reaction: When haloarenes are treated with sodium in dry ether, two aryl groups get joined together. This reaction is known as Fittig reaction.
Questions: Which reference books can one follow for aryl halides?
Answer: While covering aryl halides for JEE, one must go through the NCERT class 12th chemistry textbook part-2. A brisk walk through this chapter from NCERT not only gives you a sound idea of the various topics you need to cover but also develops the fundamentals regarding the chapter. Apart from this, you can follow a reference book to understand the mechanisms of the reactions not given in the NCERT textbook. Some authentic books for understanding the mechanism of organic reactions include books from authors like Solomons&Fryhle, Morrison & Boyd. Once you have developed concepts on this topic, you can practice questions from the books of authors like M. S. Chauhan, etc.
For further details related to aryl halides for JEE chemistry, get in touch with our mentors here at BYJU’S.


Comments