What Is Fehling’s Solution?
Fehling’s solution is a deep blue alkaline solution which is used to identify the presence of aldehydes or groups that contain any aldehyde functional group -CHO, and in addition with Tollen’s reagent to differentiate between reducing and non-reducing sugars. Fehling’s solution is also used to differentiate a ketone group and water-soluble carbohydrates.
Table of Contents
- Formation of Fehling’s Solution
- Fehling’s Test Procedure
- Reactions of Fehling’s Test
- Common Uses of Fehling’s Reagent
- Important Questions
In order to carry out Fehling’s test, the substance to be tested is heated with Fehling’s solution. The presence of any aldehyde group is indicated by the formation of a brick-red precipitate (although mild, Fehling’s solution oxidises aldehydes).
Formation of Fehling’s Solution
Fehling’s solution is prepared just before its actual use. It is done by mixing equal volumes of two previously made solutions, a deep blue Fehling’s solution A, which is 70 grams of cupric sulphate pentahydrate per litre of solution and a colourless Fehling’s solution B, which is about 350 grams of Rochelle salt (potassium sodium tartrate tetrahydrate) and 100 grams of sodium hydroxide per litre of the solution.
Also Read: Aldehyde and Ketone
The deep blue colour imparted by Fehling’s solution A is due to the bis (tartrate) complex of Cu2+. The Rochelle salt serves as a chelating agent in the solution. Both solutions, A and B, are prepared separately. They are usually kept or stored in a rubber stoppered bottle. So, Fehling’s solution is prepared usually when there is a requirement for the solution. It is made fresh in laboratories by combining equal volumes of the two mentioned solutions.
Fehling’s Test Procedure
Fehling’s test was first carried out by the German chemist Hermann von Fehling in 1849. In this test, the heating of aldehyde with Fehling’s reagent/solution is done. This process will subsequently result in the formation of a reddish-brown colour precipitate. This is because the aldehyde gets oxidised by the solution, and it further leads to the formation of a carboxylate anion.
Nonetheless, the aromatic aldehydes do not show any reaction to Fehling’s Test. Ketones also fail to react. Thus, with such properties, we can easily distinguish between ketones and aldehydes by using Fehling’s reagents.
In order to carry out Fehling’s test,
- Take the sample to be tested in a dry test tube (preferably 1 ml).
- Distilled water should be taken in another test tube for control.
- Fehling’s solutions are added to these test tubes (1 ml of each solution A and B).
- The tubes are then kept in a boiling water bath.
- Observe and record if there is any sign of the formation of the red precipitate.
The result can be concluded as positive if there is any formation of reddish-brown precipitate and can be concluded as negative if there is no indication of such change.
Solution A: CuSO4 solution.
Solution B: Rochelle salt (sodium potassium tartrate) + sodium hydroxide
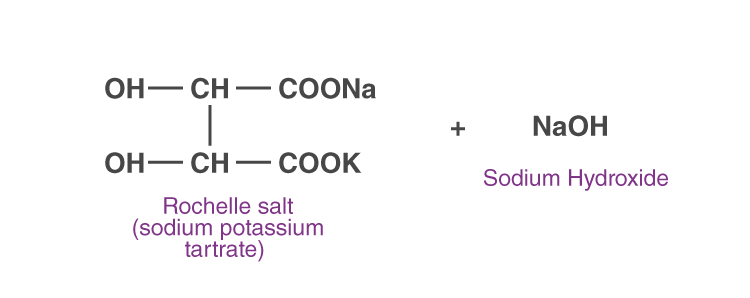
CuSO4 + 2NaOH → Cu(OH)2 + Na2SO4
Cu(OH)2 → CuO + H2O
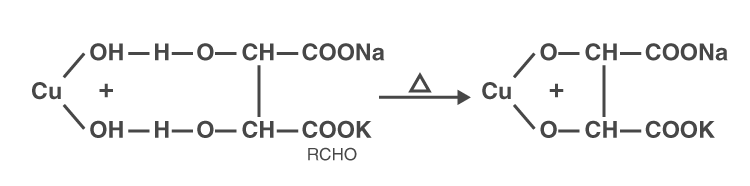
Deep blue colour complex (Fehling’s solution)
Deep blue colour → Cu2O + RCOOH
(CuO) (red ppt)
Since Fehling’s reagent is corrosive and toxic in nature, protective gloves and goggles must be worn when preparing the solution and when performing the demonstration.
Reactions of Fehling’s Test
The reaction between copper(II) ions and aldehyde in Fehling’s solution is represented as,
RCHO + 2 Cu2+ + 5 OH− → RCOO− + Cu2O + 3 H2O
When tartrate is added, the reaction can be written as:
RCHO + 2 Cu(C4H4O6)22− + 5 OH− → RCOO− + Cu2O + 4 C4H4O62− + 3 H2O
When the redox reaction is completed, the copper II ions are reduced to Copper I oxide, which forms a red precipitate and is insoluble in water. A positive test result is indicated by the presence of this red precipitate. The sodium salt of the acid is left behind in the solution.
Also Read: Redox Reactions
Common Uses of Fehling’s Reagent
- Fehling’s solution is used to distinguish between aldehyde and ketone functional groups. Aldehydes oxidise to give a positive result, but ketones won’t react to the test (except for α-hydroxy ketones).
- Fehling’s test is used as a general test for determining monosaccharides and other reducing sugars. For aldose monosaccharides, it shows a positive test result which is mainly due to the oxidisable aldehyde group. We also get a positive result for ketose monosaccharides, as they are converted to aldoses by the base in the reagent.
- In medicine, Fehling’s solution is used to detect glucose in urine as a part of detecting diabetes.
- Fehling’s reagent is used in the breakdown of starch, where it is changed to glucose syrup and maltodextrins (a polysaccharide used as a food additive). This is done in order to measure the amount of reduced sugar.
- Formic acid (HCO2H) gives a positive result for Fehling’s test.
Some Important Questions
1. What is the reason for the difference in the behaviour of aldehydes and ketones?
2. Predict the product formed when cyclohexane carbaldehyde reacts with Fehling’s reagent.
3. Write the equations for the test to distinguish between acetaldehyde and acetone.
4. Account for the following: Sodium bisulphate (Na2SO4) is used for the purification of aldehydes and ketones.
5. Give two reactions to distinguish between aldehydes and ketones.
6. An organic compound (A) with molecular formula C8H8O forms an orange-red precipitate with 2,4-DNP reagent and gives a yellow precipitate on heating with iodine in the presence of sodium hydroxide. This compound doesn’t reduce Tollens’ or Fehlings’ reagent, and it does not decolourise bromine water or Baeyer’s reagent. On excessive oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2. Determine the compounds (A) and (B), and explain the reactions involved.
7. Distinguish between the chemical compounds and provide their chemical equations.
(i) Acetaldehyde and propanone
(ii) Acetone and formaldehyde
(iii) Ethyl alcohol and acetaldehyde
(iv) Acetaldehyde and benzaldehyde
8. A compound having the molecular formula C3H6O forms a crystalline white precipitate with sodium bisulphate and reduces Fehling’s solution. Suggest the structural formula and IUPAC name of the compound. Name an isomer for it from a group other than its own.
9. Why are aldehydes more reactive towards nucleophilic reactions than ketones?
10. Write the equations of the reaction of ethanal with Fehling’s solution.


Comments