Before getting into the structure of glucose, let us first understand a few things about the compound. Glucose is a carbohydrate and an important biomolecule that helps in the metabolism of the body. It is a simple sugar having a molecular formula C6H12O6. In simple terms, we can say that it is made up of six carbon atoms, twelve hydrogen atoms and six oxygen atoms.
Glucose is a widely available monosaccharide and is also known as dextrose and blood sugar. Glucose is mainly manufactured by plants and most of the algae during the process of photosynthesis.
Table of Contents
- Different Forms of Glucose Structure
- Glucose Structure Open-Chain Formula
- Configuration of D/L-Glucose
- Cyclic Structure of Glucose
- Haworth Representation of Glucose Structure
- Physical Properties of Glucose
The glucose molecule is an important carbohydrate, as mentioned above, and it is also essential for the production of Adenosine Triphosphate (ATP), also known as the molecule of energy for the body. The level and amount of glucose are strictly regulated because both excessive and deficient amount of glucose levels results in a state of disease for the human body.
Different Forms of Glucose Structure
The structure of glucose is explained in detail with the help of the following conditions.
- Open-chain formula
- Configuration
- Cyclic structure
- Haworth representation
Glucose Structure Open-Chain Formula
The open-chain formula of glucose can be constructed with the following facts:
- Molecular formula: From the analysis of elements of glucose and from the molecular weight of glucose, the molecular formula, that is, C6H12O6, is established.
- Presence of 6-carbon unbranched chain: The proof behind glucose is a molecule made of an unbranched six-carbon chain is the fact that glucose is reduced completely when reacted with concentrated hydrogen iodide and red phosphorous giving n-hexane.
- Presence of 5 OH groups: The glucose is reacted with acetic anhydride to form pentadactyl derivatives. This simply shows the presence of five hydroxyl groups. As we know, glucose is a very stable compound; no two OH groups can be attached to the same carbon. Or in other words, we can say that the five OH groups are present on the different carbons.
- Presence of C=O group: Glucose reacts with hydroxylamine to form an oxime. It proves the presence of a carbonyl group.
- Presence of terminal CHO function: On mild oxidation of glucose with the bromine water, the glucose molecule is converted into glucose acid, which, when reduced with the high amount of HI, gives hexanoic acid.
C5H11O5.CHO→C5H11O5.COOH→CH3(CH2)4COOH
Glucose gluconic acid n-hexanoic acid
The above reaction shows that the glucose molecule contains a straight chain with CHO at one end, which has been oxidised to COOH.
- Construction of open-chain glucose structure: As you are aware that glucose has a straight 6-carbon chain with a terminal CHO, the five OH groups can be placed one each on the remaining five carbons. By supplying hydrogen atoms to these carbon atoms, it satisfies the four valencies of the carbon atom.
Configuration of D/L-Glucose
The configuration of D/L-Glucose was proved by scientist Emil Fischer by arguments similar to the ones stated below.
Construction of four possible D-pentoses: Taking the configuration of D-glyceraldehyde as the standard, two possible D-aldotetroses may be constructed by adding a CHOH just below CHO by placing Oh to the right and after it to the left.

D-Arabinose has configuration II or IV: Oxidation of D-arabinose with nitric acid oxidises the terminal CHO and CH2OH groups yielding two optically active dicarboxylic acids. Both II and IV can form two optically active diacids, while I and III can give meso acids only that have a plane of symmetry. Therefore, D-arabinose is either II or IV.
Also Read: Fructose
Ruff degradation of D-glucose and D-mannose produces D-arabinose in each case: In ruff degradation, the CHOH below CHOH is destroyed. Therefore, the configuration of two aldohexoses, D-glucose and D-mannose, can be derived by adding a new CHOH below CHO in form II of D-arabinose.

Hence, D-glucose has configuration V or VI.
D-glucose and L-glucose yield the same dicarboxylic acid: This means that these two sugars differ only with respect to the position of the terminal groups (CHO and CH2OH). Therefore, the exchange of the terminal groups in D-glucose should be able to give a different aldohexose.

If VII is rotated through 180⁰C in the plane of the paper, it gives an aldohexoses VIII, different from V. A similar procedure with formula VI does not give rise to a different sugar.
Cyclic Structure of Glucose
Open-chain structure not wholly true: Fischer realised that the open-chain pentahydroxy aldehyde structure of glucose did not wholly explain its chemical behaviour. Unlike simple aldehydes, glucose did not form the crystalline bisulphate compound and failed to give Schiff’s test. Furthermore, the pentaacetate and pentamethyl ether derivatives of glucose are not oxidised by Tollens reagent or Fehling’s solution, indicating the absence of the CHO group.
The cyclic structure suggested explaining mutarotation: The French chemist, Tarnet established the existence of two crystalline forms of glucose, alpha-glucose and beta-glucose; alpha-glucose has specific rotation +112⁰, while beta-glucose +19⁰. The optical rotation of each of these forms changed gradually with time till a constant value of +53⁰ was reached.
To explain this phenomenon of mutarotation, it was visualised that the alpha and beta glucose were, in reality, the cyclic hemiacetal forms of glucose, which were interconvertible via the open-chain form. The constant value of +19⁰ represented the state of equilibrium between alpha-D-glucose and beta-D-glucose.

Glycoside formation confirms cyclic structure: When we use methanol in the presence of dry HCl to treat glucose, it gives two isomeric glycosides or acetals. These crystalline glycosides, namely methyl-alpha-D-glucose and methyl-beta-glucoside, are actually isolated. These are optically active but do not give any reactions of the free CHO group. Evidently, the two glycosides are the methyl derivatives of alpha and beta-D-glucose, formed as a result of the reaction between the hemiacetal OH of these forms and methanol.

Thus, the cyclic structure of D-glucose stands confirmed, but whether it has a 5–membered or 6-membered ring is still to be proved.
Determination of ring size: So far, we have represented the structure of cyclic hemiacetals or anomers of D-glucose as having a ring of six members, five carbons and one oxygen. This has been proven to be correct, and the five-membered ring has been ruled out.
Hirst, in 1926, prepared tetra-O-methyl-D-glucose by treating methyl-D-glucoside with dimethyl sulphate and subsequent acid hydrolysis of the pentamethyl derivative formed. The oxidation of tetra-O-methyl-D- glucose with nitric acid yielded trimethoxy glutaric acid.

Obviously, the two carboxylic carbons (1,5) of the trimethoxy glutaric acid are the ones originally involved in ring formation. Hence, there must have existed an oxide ring between C-1 and C-2. Tracing back the reaction sequence, it stands proved that D-glucose has a six-membered ring. The presence of a 6-membered ring in D-glucose has also been confirmed by X-ray analysis.
The Haworth Representation of Glucose Structure
We have already talked about Fischer projection formulas for representing the cyclic forms of D-glucose. However, Haworth thought that these structures were awkward. He developed the hexagonal representations, which resembled the heterocyclic pyran containing five carbon and one oxygen in the ring. He came up with the names alpha-D-glucopyranose and beta-D-glucopyranose for the hexagonal structures of alpha-D-glucose and beta-D-glucose.
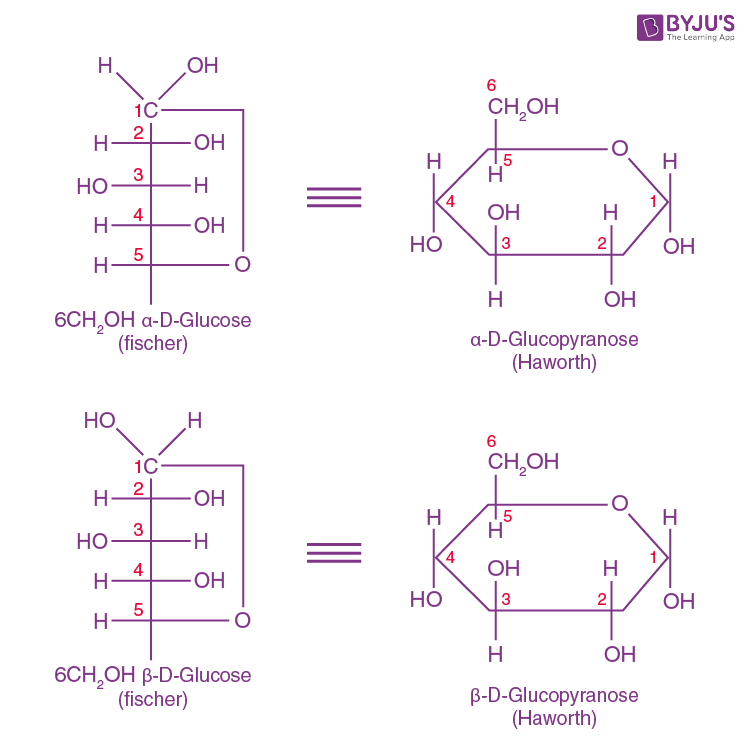
It may be noted that in the Haworth formula, all the OH groups on the right in the Fisher formula are directed below the plane of the ring, while those on the left go above the plane of the ring. The terminal CH2OH projects their OH group above the plane of the ring of a glucose molecule.
Also, Read: Sucrose
Physical Properties of Glucose
Glucose is a white crystalline solid with a melting point of 146⁰C. When this glucose molecule is crystallised with cold water, it forms glucose monohydrate having molecular formula C6H12O6.H20 and a melting point of 86⁰C. It is extremely soluble in water, only sparingly so in ethanol, and insoluble in ether. It is about three-fourths as sweet as cane sugar that is sucrose. It is optically active, and the ordinary naturally occurring form is (+)- glucose.

Comments