The hybridization of XeF2 (Xenon Difluoride) is an sp3d type. Here we will try to understand all the steps involved and how to determine this type of hybridization.
| Name of the Molecule | Xenon Difluoride |
| Molecular Formula | XeF2 |
| Hybridization Type | sp3d |
| Bond Angle | 180o |
| Geometry | Linear |
What is the Hybridization of Xenon Difluoride?
In the hybridization of xenon difluoride, Xenon (Xe) is the central atom. Now if we count the number of valence shell in Xe we will find two electrons in the 5s orbital and six electrons in the 5p orbital. It’s ground state electronic configuration will be 5s2 5p6. However, in the excited state, its configuration will change to 5s2 5p5 5d1. All in all, in the excited state configuration 5s,5py, 5px, 5pz,5dzx atomic orbitals of the xenon atom will be hybridized to forms 5 sp³d hybrid orbitals.
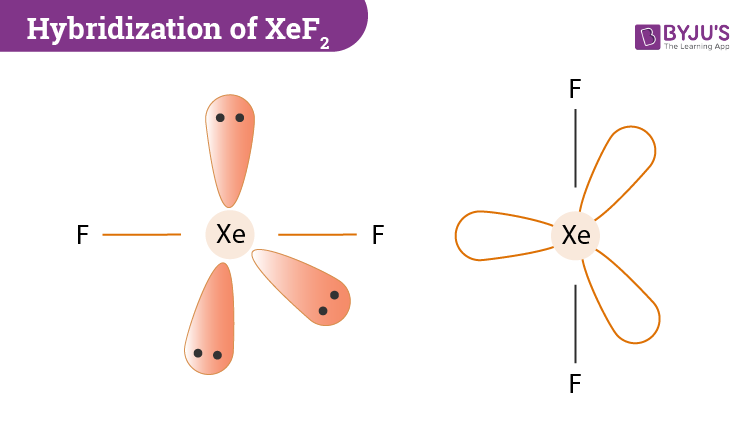
Two-hybrid orbitals are used in the formation of F-Xe-F sigma bonds by overlapping the two half-filled 2pz atomic orbitals of fluorine. The other remaining three hybrid orbitals contain the lone pairs which do not participate in bonding.
Important Points To Remember
- The central atom Xe has 2 bond pair and 3 lone pairs around it.
- During hybridization, Xenon will form two sigma bonds with two fluorine atoms.
- There are three hybrid orbitals that contain the lone pairs and they do not form any bonds.
XeF2 Molecular Geometry And Bond Angles
XeF2 molecular geometry is linear. It acquires such shape as the lone pairs present around the central atom tend to take up equatorial positions. The bond angle is said to be 180°.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of BCl3
- Hybridization Of BrF5
- Hybridization Of XeO2F2
- Hybridization Of BeCl2
- Hybridization Of Ethene

Comments