Paramagnetic materials are materials that tend to get weakly magnetized in the direction of the magnetizing field when placed in a magnetic field. Paramagnetic materials have a permanent dipole moment or permanent magnetic moment. However, if we remove the applied field, the materials tend to lose their magnetism. This is because thermal motion randomizes the spin orientations of the electrons.
Download Complete Chapter Notes of Magnetism and matter
Download Now
Table of Contents
- What Are Paramagnetic Materials?
- Properties of Paramagnetic Materials
- Paramagnetic Liquid Demonstration
- Important Points to Remember
- Paramagnetic Materials Examples
- Superparamagnets
- Frequently Asked Questions – FAQs
What Are Paramagnetic Materials?
Paramagnetic materials have some unpaired electrons, and due to these unpaired electrons, the net magnetic moment of all electrons in an atom is not added up to zero. Hence, an atomic dipole exists in this case. On applying an external magnetic field, the atomic dipole aligns in the direction of the applied external magnetic field. In this way, paramagnetic materials are feebly magnetized in the direction of the magnetizing field.
Also Read: Ferromagnetic Materials
In simple words, we can say that these materials usually experience a weak attraction to magnets. This type of magnetism is known as paramagnetism. It occurs mainly due to the presence of unpaired electrons in the material or due to the partial alignment of randomly oriented atomic dipole along the field.
Further, paramagnetism can be of two types:
- In the first type, the magnetic moments are found in low concentrations, which leads to their separation from one another. Their spins also do not interact.
- In the second type, paramagnetism occurs due to the interactions between the magnetic moment. In this case, the interactions are very weak. Therefore, there is no net magnetization when the applied field is zero.
This type of magnetization is based on Curie’s law. According to the law, paramagnetic materials’ magnetic susceptibility χ is inversely proportional to their temperature. It is represented as,
M = χH = C/T x H
Where,
M = magnetization,
χ = magnetic susceptibility,
C = material-specific Curie constant,
T = absolute (Kelvin) temperature,
H = auxiliary magnetic field.
Properties of Paramagnetic Materials
- When the net atomic dipole moment of an atom is not zero, the atoms of paramagnetic substances have permanent dipole moment due to unpaired spin.
- The substances are weakly attracted by the magnetic field.
- In the non-uniform external magnetic field, paramagnetic substances move from a weak field region to a strong field region.
- A paramagnetic rod sets itself parallel to the field because the field is strongest near the poles.
- A paramagnetic liquid in a U-Tube ascends in the limb, which is between the poles of the magnet.
- The intensity of magnetization is very small, positive and directly proportional to the magnetizing field.
- Magnetic susceptibility is small and positive.
- The relative permeability is slightly greater than 1. The field inside the material is greater than the magnetizing field.
- Magnetic field lines become denser inside paramagnetic substances.
- Magnetization of paramagnetic substances is inversely proportional to absolute temperature.
- Paramagnetic substances obey Curie’s law, according to which magnetic susceptibility is inversely proportional to its absolute temperature.
- The magnetic dipole moment of paramagnetic substances is small and parallel to the magnetizing field.
Paramagnetic Liquid Demonstration
If a paramagnetic liquid is placed in a watch glass placed on two pole pieces which are quite close to each other, then the liquid accumulates in the middle where the field is strongest.
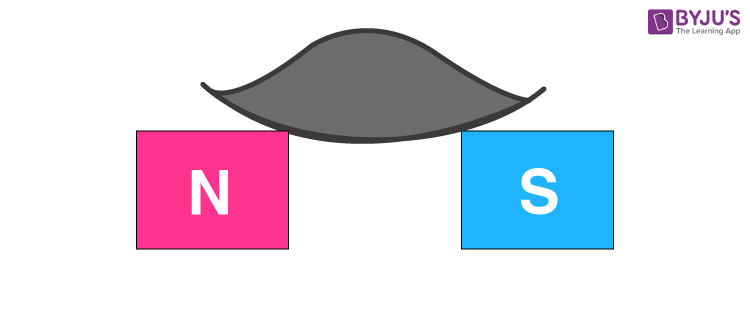
If a paramagnetic liquid is placed in a watch glass placed on two pieces which are sufficiently apart, then liquid accumulates at the sides and shows depression in the middle because the field is strongest at the poles.
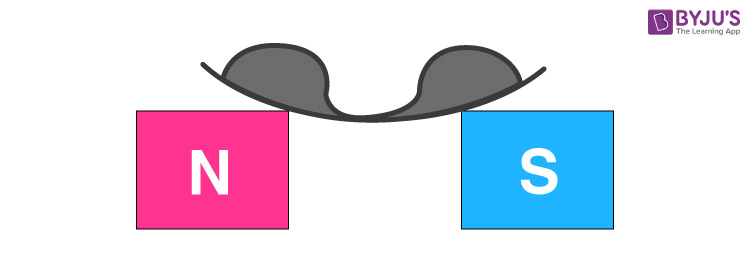
Important Points to Remember
- The magnetization of paramagnetic substances is inversely proportional to absolute temperature. It means that if we increase the temperature, paramagnetic substances start losing their magnetic power.
- On lowering the temperature, a stage comes when 100℅ atomic dipoles align with the external magnetic field; this state is called a saturation state, and after this, Curie’s law is invalid.
- Paramagnetism is executed by solids, liquids and gases.
Also Read: Diamagnetic Materials
Paramagnetic Materials Examples
At this point, we have learnt that materials that show paramagnetism are paramagnetic. Nonetheless, true paramagnets are those materials that show magnetic susceptibility with respect to the Curie law. They also show paramagnetism regardless of the temperature range. Some examples of paramagnetic materials include iron oxide, oxygen, magnesium, molybdenum, titanium, lithium, aluminium, transition metal complexes, etc.
| Material | Magnetic Susceptibility, [10−5]
(SI Units) |
| Tungsten | 6.8 |
| Caesium | 5.1 |
| Aluminium | 2.2 |
| Lithium | 1.4 |
| Magnesium | 1.2 |
| Sodium | 0.72 |
Superparamagnets
Superparamagnets are the elements that not only show a net paramagnetic response but also tend to exhibit strong ferromagnetic or ferrimagnetic ordering mostly at a microscopic level. These materials also follow Curie’s law and have very large Curie constants. Ferrofluids are superparamagnets.
Frequently Asked Questions on Paramagnetic Materials
Explain what paramagnetic materials are.
Due to the presence of unpaired electrons in paramagnetic materials, the net magnetic moment of all electrons in an atom does not equal zero. As a result, atomic dipoles exist. The atomic dipoles align in the direction of the applied external magnetic field when it is applied. Paramagnetic materials are weakly magnetised in the direction of the magnetising field.
Give examples of paramagnetic substances.
Tungsten, Caesium, Aluminium, Lithium, Magnesium and sodium are a few examples of paramagnetic substances.
What is paramagnetism?
The magnetic condition of an atom having one or more unpaired electrons is known as paramagnetism.

Comments