Saytzeff’s Rule is also called Zaitsev’s rule, Saytzev’s rule or Z-rule. A Russian chemist, Alexander Zaitsev, analysed different elimination reactions and observed a general pattern in the resulting alkenes. Based on this analysis, Zaitsev stated that a stable alkene is formed when the removal of hydrogen from β-carbon has a low number of hydrogen substituents.
Download Complete Chapter Notes of Hydrocarbons
Download Now
During elimination reactions, Saytzeff’s rule comes into the picture. The most substituted product would be the most stable and preferred one. This rule does not generalise the product stereochemistry but only the regiochemistry of the elimination reaction.
Also read: Elimination Reaction
Elimination reactions of some alkyl halides and alcohol will result in different alkenes, and Saytzeff’s rule is used to predict the major product. The major and minor products are predicted based on the number of alkyl groups attached to the alkene.
Important Concept Behind Saytzeff’s Rule
- If more than one elimination product is possible, the most substituted alkene is the most stable product (major product).
- CH2 = CHR < RCH = CHR < R2C = CHR < R2 C = CR2
Mono < di < Tri < Tetra
Examples:
1. Dehydrohalogenation of 2-Bromobutane
.
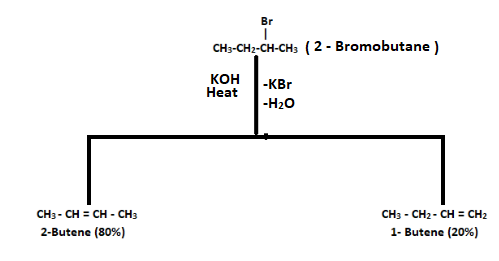
When 2-bromobutane undergoes a dehydrohalogenation reaction, it gives two products: 1-butene and 2-butene. Out of these two, 2-butene is a major product since it is highly substituted and more stable.
2. Dehydration of Alcohol
When butanol undergoes dehydration, it gives two products: 1-butene (minor product) and 2-butene (major product).
Mechanism of Elimination Reaction
Consider a dehydration reaction of 2, 3-dimethylpentan-3-ol
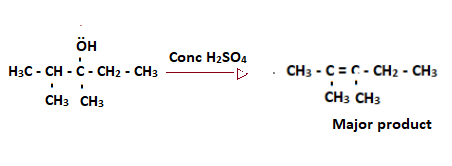
Step 1:
Sulfuric acid ionizes to give a proton
H2SO4 → H+ + HSO4–
Step 2:
Proton formed in step 1 reacts with the OH group and forms OH2+, and the loss of water gives a positive charge on the carbon atom and forms a tertiary carbocation.
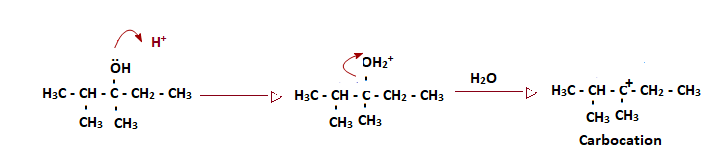
Step 3:
Removal of hydrogen adjacent to the tertiary carbocation gives alkene (Double bond formation). Three alkene formations are possible, as shown below:
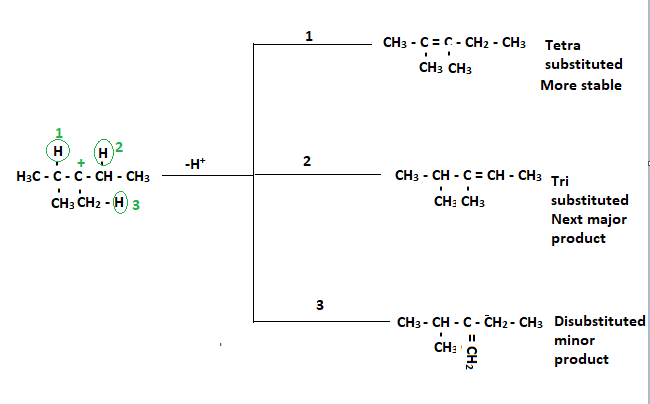
Summary:
- Saytzeff’s rule predicts the regioselectivity of the olefin (alkene) formed by the elimination reaction of 2o or 3o alkyl halides.
- During the elimination reaction, the proton is removed from the carbon atom having less number of substituents.
- The corresponding olefin is known as Saytzeff’s product.
General Organic Chemistry



Comments