What Are Surfactants?
Surfactants are a category of chemical compounds that are used in lowering the surface tension (or interfacial tension) between different compounds, such as two liquids or between a gas and a liquid, or it can also be between a liquid and a solid. Surfactants are categorised as organic compounds and are amphiphilic in nature. It basically means that they contain both hydrophobic and hydrophilic groups.
In other words, a surfactant has both a water-insoluble component and a water-soluble component. One of the common properties of surfactants is that they will diffuse in water and adsorb at interfaces between air and water. It can also adsorb at the interface between oil and water where water is mixed with oil. The water-insoluble group can extend out of the bulk water phase and move into the air or oil phase. On the other hand, the water-soluble head group usually stays in the water phase.
The word surfactant has been derived from the word surface active agent and was coined in the year 1950. In any case, surfactants today are found in many products that we use today. They are found in detergents, wetting agents, foaming agents, emulsifiers and dispersants. Surfactants are one of the important components in detergents that help to eliminate dirt from clothes, skin and household utensils, mainly in bathrooms and kitchens. Besides, they are extensively used in industries.
Soaps are the foremost surfactants, mostly obtained from fats called glycerides, which are mainly esters formed by trihydric alcohol, glycerol, with fatty acids having lengthy chain carboxylic acids. Glycerides are hydrolysed by heating with a solution of sodium hydroxide to make soaps, sodium salts of acids and propane 1,2,3-triol. This process is called saponification.
How Do Surfactants Work?
When a sufficient amount of surfactant molecules are added to a solution, they start combining together. During this, they form structures or aggregates called micelles in the bulk aqueous phase. As the micelle starts to form, the surfactant heads (hydrophilic heads) remain exposed to water or the surrounding liquid. The tails (hydrophilic heads) come together at the centre of the structure and remain protected from water. Different types of aggregates, such as spherical or cylindrical micelles or lipid bilayers, can also be formed. Furthermore, the shape of the aggregates mostly depends on the chemical structure of the surfactants (balanced size between the hydrophilic head and hydrophobic tail).
In any case, surfactants work by breaking down the interface between oils, water or dirt. The oils and dirt are also held in suspension, making it easy to remove them.
Action of Surfactants
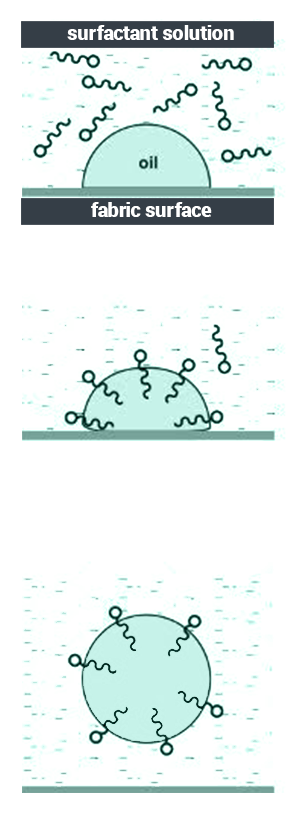
- Surfactants consist of hydrophobic (water-hating) and hydrophilic (water-loving) groups.
- The molecules of surfactant are adsorbed by the oil, and hence they are removed from the surface.
- The molecules of surfactant surround the oil after it has been removed and prevent it from depositing again.
Manufacture
The glycerides which are used in making surfactants contain unsaturated and saturated carboxylic acids that consist of an even number of carbon atoms in the range of 12-20, such as stearic acid, CH3(CH2)16CO2H. Synthetic surfactants hold one major edge over soaps. Since soaps form insoluble magnesium and calcium salts with magnesium and calcium ions in hard water and clays that exist in the dirt where a lot of soap goes in vain in making an insoluble scrum. However, this can be avoided by using a synthetic surfactant. For instance, in anionic surfactants, the carboxylate group is replaced by sulfonate as the hydrophilic component. The corresponding magnesium and calcium salts are soluble in water more than the salts of carboxylic acids.
Types of Surfactants
There are several types of surfactants that are classified according to the polar head group. The hydrophobic tails are seen to be often similar. Let’s quickly go through them below.
Anionic
If the charge on the head group (hydrophilic end) is negative, the surfactant is called anionic. It contains anionic functional groups at its head, such as sulfate, sulfonate, phosphate, and carboxylates. A few examples of anionic surfactants include sulfates, sulfonates, and gluconates.
Cationic
Similarly, if the head group (hydrophilic end) has a positive charge, it is called cationic. Alkyl ammonium chlorides are common examples of cationic surfactants.
Zwitterionic
Zwitterionic, also known as amphoteric surfactants, have both positive and negative charges on their hydrophilic end. They have both cationic and anionic centres attached to the same molecule. It basically has a net charge of zero. Betaines and amino oxides are examples of this type of surfactant.
Non-ionic
Nonionic surfactants are mostly neutral, and no charge is present on their hydrophilic end. Non-ionic surfactants have covalently bonded oxygen-containing hydrophilic groups, which are bonded to hydrophobic parent structures. They can be used to emulsify oils and seem to do a better job than anionic surfactants at removing organic soils. Non-ionic surfactants are less sensitive to water hardness than anionic surfactants, and they foam less strongly. Some common examples of nonionic surfactants are ethoxylates, alkoxylates and cocamide.
Uses of Surfactants
A vast range of surfactants like emulsifiers, foaming agents and wetting agents are utilised based on the areas of use. Surfactants minimise the surface tension with respect to the phase and hence lie at the heart of interfacial chemistry.
- Surfactants play an important role in cleaning, wetting, dispersing, emulsifying, foaming and anti-foaming agents.
- Used in agrochemical formulations such as herbicides (some), insecticides, biocides (sanitisers), and spermicides.
- Used in personal care products such as cosmetics, shampoos, shower gel, hair conditioners, and toothpaste.
- Surfactants are used in firefighting and pipelines (liquid drag-reducing agents). Alkali surfactant polymers are also used to mobilise oil in oil wells.
- Surfactants are sometimes added to car engine lubricants which greatly help to keep particles from sticking to engine parts.
- Surfactants are also commonly used in corrosion inhibition in ore flotation.
- They are also used in corrosion inhibition, to promote oil flow in porous rocks, and to produce aerosols.
You can visit BYJU’S to know more about the applications of surfactants in daily life and manufacturing processes in various industries.

Comments