Lithium iodide is a chemical compound composed of lithium and iodine, which when exposed to air turns yellow due to the oxidation of iodide to iodine. It has a chemical formula LiI. In this article, we shall be discussing the lithium iodide formula along with its chemical structure and formula and few of the lithium iodide uses.
Lithium Iodide Properties
| Properties of Lithium Iodide | |
| Name | Lithium Iodide |
| Appearance | White crystalline powder |
| Molecular Formula | LiI |
| Melting Point | 469 oC |
| Boiling Point | 1171 oC |
| Density | 4.08 g/cm³ |
| Molar Mass | 133.85 g/mol |
| Solubility in Water | Soluble |
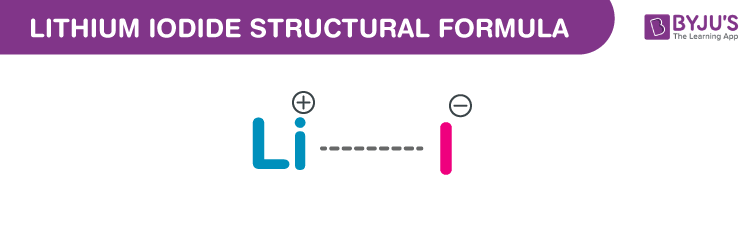
Lithium Iodide Chemical Structure
Lithium Iodide Uses
- Used as an electrolyte for a high-temperature battery
- In organic synthesis, it is useful for cleaving C–O bonds
- Used as a phosphor for neutron detection
To learn more about such chemistry topics register to BYJU’S now!
Comments