Magnesium carbonate is a white inorganic salt that is odourless with several industrial uses. The magnesium carbonate formula is MgCO3. It is slightly more alkaline than acidic. The most common magnesium carbonate form is the anhydrous salt known as the magnesite. Magnesite consists of white trigonal crystals. Magnesium carbonate is commonly obtained by mining the mineral magnesite. Seventy per cent of the world’s supply is mined and prepared by China. In this short piece of article, let us learn more about the magnesium carbonate formula, chemical structure and its general properties.
Magnesium Carbonate Properties
| Magnesium Carbonate Properties | |
| Name | Magnesium Carbonate |
| Appearance | White, yellowish, greyish-white or brown crystalline powder |
| Molecular Formula | MgCO3 |
| Melting Point | 350 °C (anhydrous)
165 °C (trihydrate) |
| Density | 2.96 g/cm³ (anhydrous)
2.825 g/cm3 (dihydrate) 1.837 g/cm3 (trihydrate) 1.73 g/cm3 (pentahydrate) |
| Molar Mass | 84.3139 g/mol (anhydrous) |
Magnesium Carbonate Chemical Structure
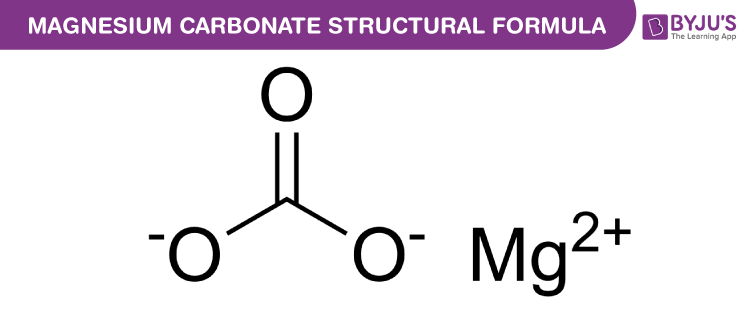
Magnesium Carbonate Uses
- Used in the production of magnesium oxide
- Used in cosmetics and toothpaste
- Used in fireproofing, flooring and fire extinguishing compositions
- Used in taxidermy for whitening skulls
To learn more about such chemistry topics register to BYJU’S now!
Comments