Mercury (II) chloride formula, also named as Mercury dichloride formula or Mercuric chloride formula is discussed in this article. It consists of one mercury (II) cation (Hg+2) and two chloride anions (Cl-1). The molecular or chemical formula of Mercury (II) chloride is HgCl2.
Mercuric chloride cannot be obtained naturally and therefore is produced chemically. It is crystalline solid white in colour. It has no odour. It is readily soluble in hot water, ethyl acetate, acetone, and ethanol but poorly soluble in cold water. It slightly dissolves in benzene and pyridine. The other names of mercury (II) chloride are mercury dichloride, mercuric chloride, corrosive sublimate.
Mercury (II) chloride Formula Structure
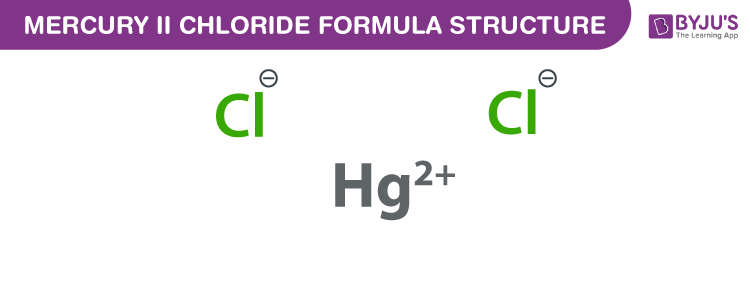
Properties Of Mercury (II) chloride Formula
| Chemical Formula | HgCl2 |
| Molecular Weight | 271.52 g/mol |
| Density | 5.43 g/cm3 |
| Boiling Point | 304 °C |
| Melting Point | 276 °C |
Safety Measures
- It is an extremely toxic compound
- It is considered fatal when it comes in contact with the skin, eyes and causes severe damage.
To learn more about Mercury (II) chloride formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Mercury dichloride for free.
Comments