Knowing the Madhya Pradesh Class 12 Revised Chemistry Syllabus help the student to plan their studies in a better way. This MP Board 12th Chemistry Syllabus was devised with the help of the Madhya Pradesh Board. The Madhya Pradesh Board offers a wide range of topics in Chemistry for Class 12. Whether you want to pursue a career in engineering or other fields related to the subject, such as Pharmaceuticals, you will have the perfect foundation to pursue higher studies, in this Madhya Pradesh Board Class 12 Chemistry Revised Syllabus 2021-22.
We have included Madhya Pradesh Board Class 12 Chemistry Syllabus 2021-2022, so you can start your preparations in earnest. From traditional Science topics such as Electrochemistry to more modern topics such as Polymers, the exhaustive list of Chemistry syllabus is all that you need to prepare for Madhya Pradesh Board Class 12 Chemistry exams.
The 2021-22 syllabus has undergone revision the syllabus reduced for this academic year, to make up for the academic losses as a result of the Covid-19 pandemic.
MP Board Class 12 Reduced Chemistry Syllabus 2021-2022 PDF
Here, we have given the screenshot of the recent syllabus chapter names in Hindi:
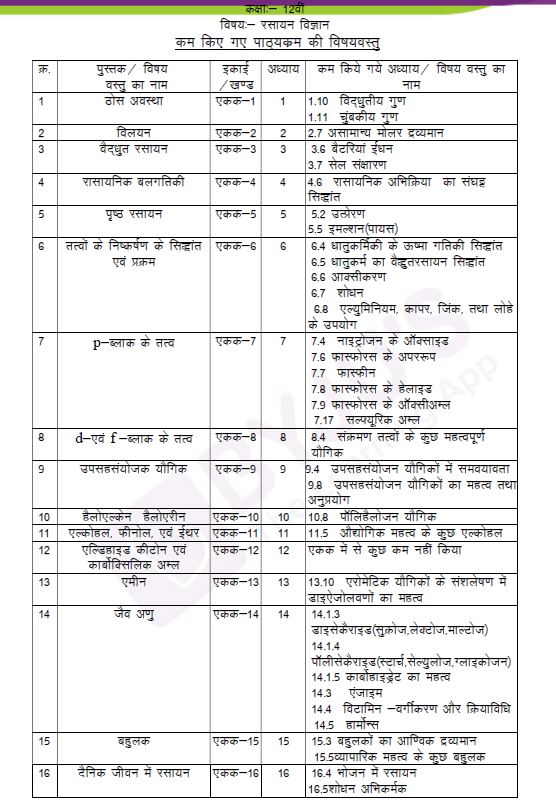
Meanwhile, find also details of the Chapter names with the deleted portions from the 2020-21 syllabus, for the previous academic year.
Unit I Solid State
Unit II Solutions
Unit III Electrochemistry
Unit IV Chemical Kinetics
Unit V Surface Chemistry
Unit VI General Principles and Processes of Isolation of Elements (deleted for 2020-21 syllabus)
Unit VII p -Block Elements
Unit VIII d -and f -Block Elements
Unit IX Coordination Compounds
Unit X Haloalkanes and Haloarenes
Unit XI Alcohols, Phenols and Ethers
Unit XII Aldehydes, Ketones and Carboxylic Acids
Unit XIII Amines
Unit XIV Biomolecules
Unit XV Polymers (deleted for 2020-21 syllabus)
Unit XVI Chemistry in Everyday Life
Students can also refer to other resources like the mp board model paper class 12th hindi medium 2020 to prepare for the exams. Stay tuned for more updates about mp board 12th.

Comments