Flashcards for NEET Chemistry are designed to boost your NEET preparation. Find below flashcards for the chapter “Hydrogen”. These flashcards are prepared as per the NEET syllabus. These are helpful for aspirants of NEET and other exams during last-minute revision. It covers all the important points that are frequently asked in the exam. Check BYJU’S for the full set of Flashcards and Study material for NEET Chemistry.
Download PDF of NEET Chemistry Flashcards for Hydrogen
|
Name of the NEET Sub-section |
Topic |
Flashcards Helpful for |
|
Chemistry |
Hydrogen |
NEET Exams |
|
Hydrogen |
|
|
Dihydrogen (H2) |
The most abundant element in the universe 70% of the total mass of the universe 0.15% by mass in the earth’s atmosphere |
|
Isotopes of Hydrogen |
Protium – 11H (No neutrons) Deuterium (Heavy hydrogen) – 12H (One neutron) Tritium – 13H (Two neutrons) |
|
Laboratory Preparation of Dihydrogen |
By the reaction of zinc with dilute hydrochloric acid Zn + 2H+ → Zn2+ + H2 By the reaction of zinc with aqueous alkali Zn + 2NaOH → Na2ZnO2 + H2 |
|
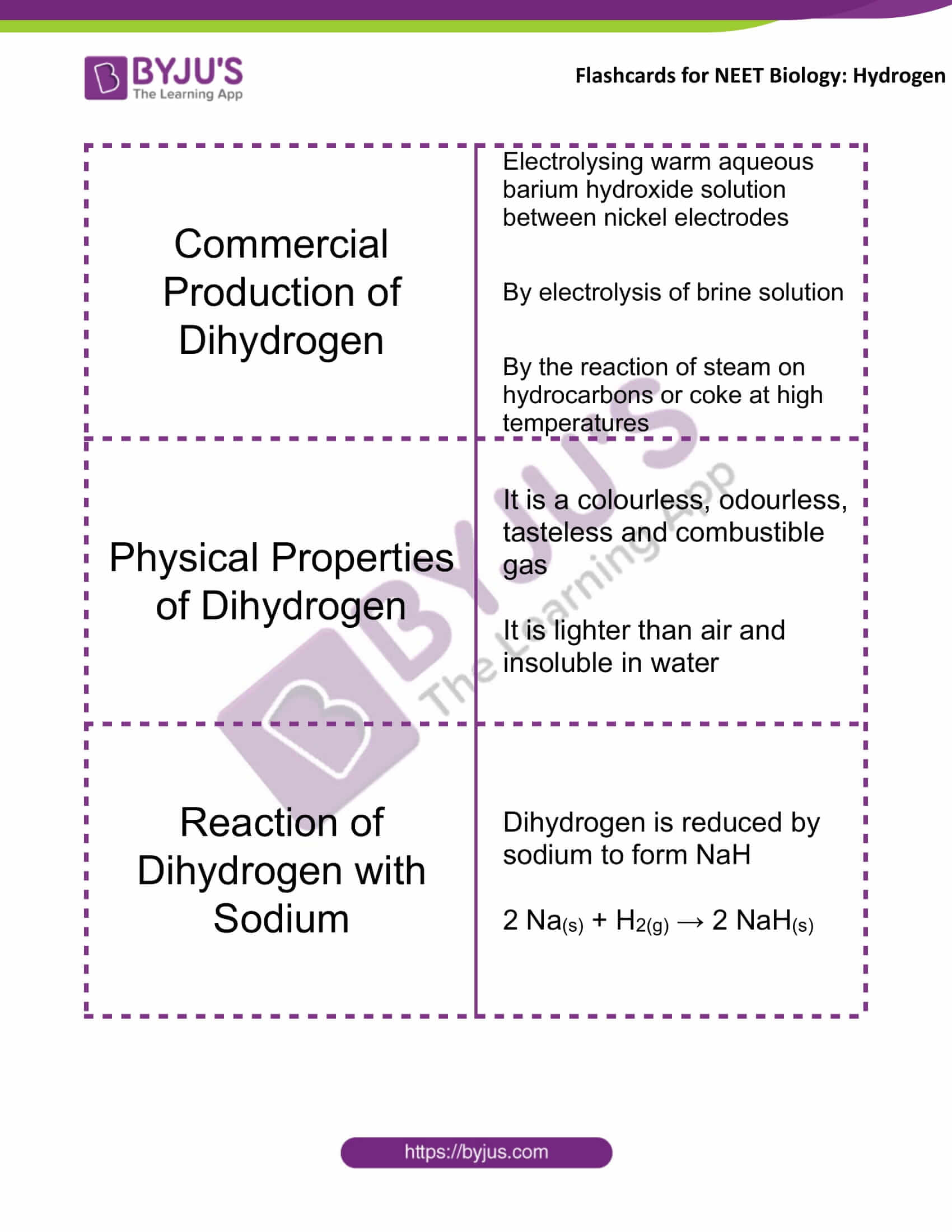
Commercial Production of Dihydrogen |
Electrolysing warm aqueous barium hydroxide solution between nickel electrodes By electrolysis of brine solution By the reaction of steam on hydrocarbons or coke at high temperatures |
|
Physical Properties of Dihydrogen |
It is a colourless, odourless, tasteless and combustible gas It is lighter than air and insoluble in water |
|
Reaction of Dihydrogen with Sodium |
Dihydrogen is reduced by sodium to form NaH 2 Na(s) + H2(g) → 2 NaH(s) |
|
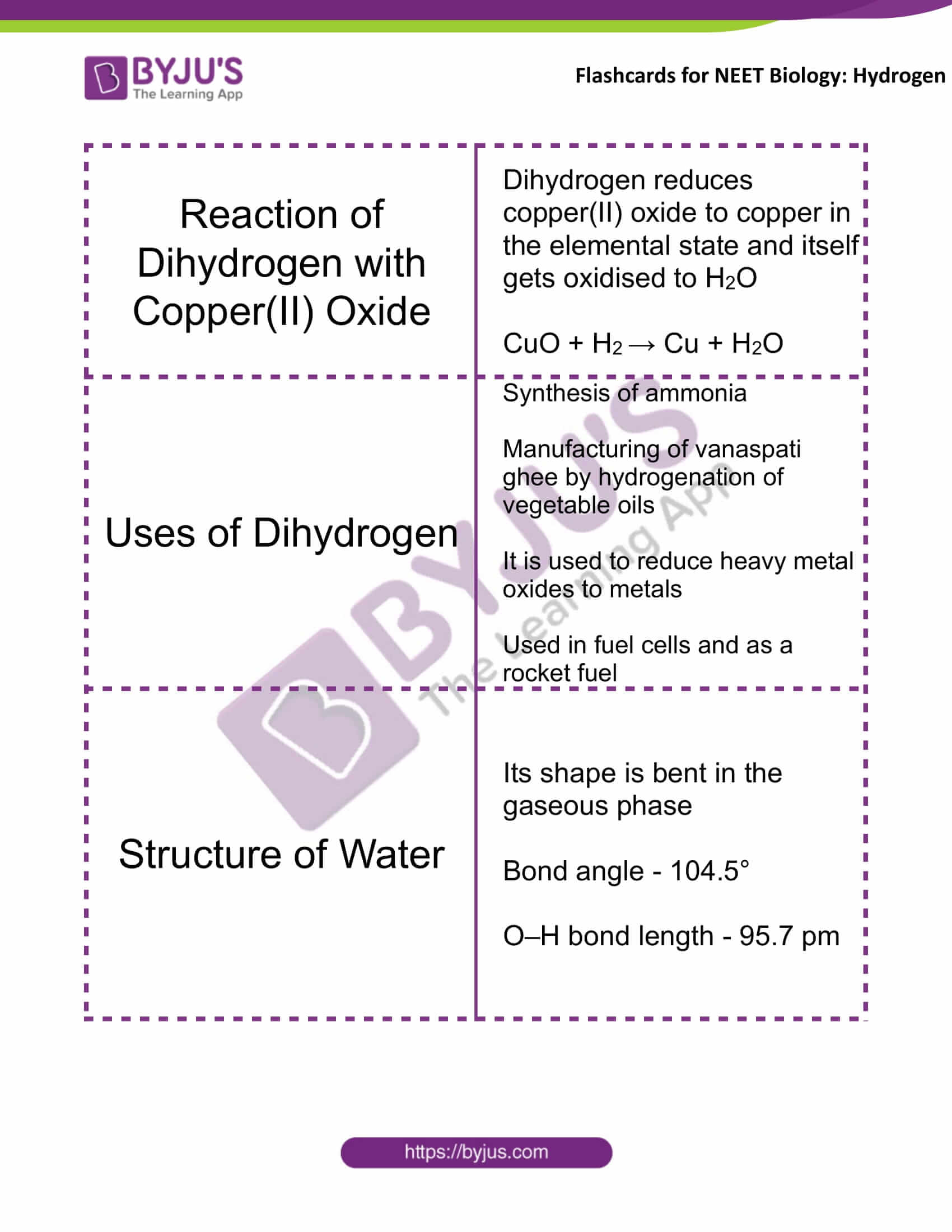
Reaction of Dihydrogen with Copper(II) Oxide |
Dihydrogen reduces copper(II) oxide to copper in the elemental state and itself gets oxidised to H2O CuO + H2 → Cu + H2O |
|
Uses of Dihydrogen |
Synthesis of ammonia Manufacturing of vanaspati ghee by hydrogenation of vegetable oils It is used to reduce heavy metal oxides to metals Used in fuel cells and as a rocket fuel |
|
Structure of Water |
Its shape is bent in the gaseous phase Bond angle – 104.5° O–H bond length – 95.7 pm |
|
Hard Water |
Hardness is due to the presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate |
Get access to the full set of flashcards for NEET Chemistry, only at BYJU’S.
Recommended Video:
Hydrogen Class 11 and 12 Chemistry One Shot (Formula + Questions)

|
Also Check: |


Comments