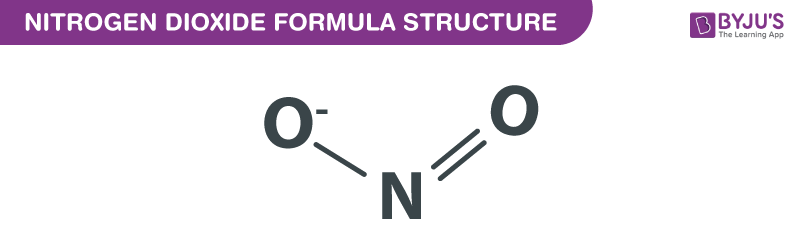
Nitrogen Dioxide formula is explained in this article. It is an extremely poisonous gas. It can function as an inhibitor. It is a pollutant which absorbs UV light and does not allow it to come to the earth’s surface. This chemical compound is paramagnetic consisting of two oxygen atoms connected to a nitrogen atom. The bond angle 134.4° whereas the distance between nitrogen and oxygen is 119.7 pm. The chemical or molecular formula of Nitrogen Dioxide is NO2.
In its gaseous state, it appears as a reddish-brown gas. In its liquid state, it appears as a yellowish-brown coloured compound. It has a pungent to acrid smell. It is toxic when inhaled and absorbed through the skin. Non-combustible, but enhances the burning of combustible materials.
Nitrogen Dioxide Formula Structure
Properties Of Nitrogen Dioxide Formula
| Chemical formula of Nitrogen Dioxide | NO2 |
| Molecular weight of Nitrogen Dioxide | 46.006 g/mol |
| Density of Nitrogen Dioxide | 1.880 g/dm3 |
| Boiling point of Nitrogen Dioxide | 21.15 °C |
| Melting point of Nitrogen Dioxide | −9.3 °C |
It is obtained by the process of combustion in the presence of air. Air acts as an oxidant. The most common sources of nitrogen dioxide are internal combustion engines, pulp mills, thermal power stations, etc. Exposure to this compound can be fatal. Contact may cause frostbite and burns to eyes and skin.
To learn more about Nitrogen Dioxide formula from the expert faculties at BYJU’S, register now!
Comments