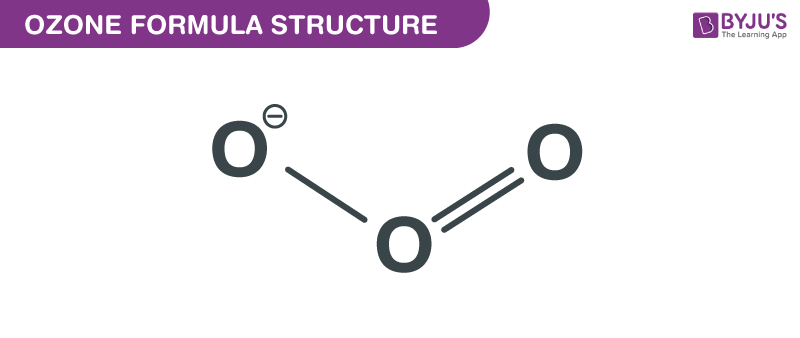
Ozone formula, also known as Trioxygen formula or Triatomic Oxygen formula is explained in this article. This inorganic molecule is an allotrope of oxygen. It consists of three atoms of oxygen, with one single bond, one double bond and an additional of two partial charges viz a negative charge and a positive charge. The “V” angle between the central carbon and the other two carbons is 116.78º. The chemical or molecular formula of Ozone is O3.
Trioxygen is a pale blue to colourless gas and has a pungent, sharp smell. It has no smell but has a bitter, hard, saline taste. It is insoluble in water but it is soluble in sulfuric acid and carbon tetrachloride. Ozone is generated from dioxygen due to the action of electrical discharges and ultraviolet light within the atmosphere of the earth.
Ozone Formula Structure
Properties Of Ozone
| Chemical formula | O3 |
| Molecular weight | 47.997 g/mol |
| Density | 2.144 mg/cm3 |
| Boiling point | −112 °C |
| Melting point | −192.2 °C |
Safety Measures
- This inorganic gas compound can cause eyes and skin irritation.
- It can cause genetic defects if inhaled or ingested.
- It is a strong oxidant and is highly toxic to the aquatic habitat.
To learn more about Ozone formula from the expert faculties at BYJU’S, register now!
Comments