Electron emission is the ejection of an electron from the surface of matter. We know that the electrons are attracted to the protons in the nucleus of an atom. It is this attraction that holds the electrons in place. But if the electrons gain sufficient energy from an external source, the electrons can escape the metal surface. In this article, we will explore the definition and the types of electron emission.
| Table of Contents |
What is Electron Emission?
Electrons are negatively charged sub-atomic particles responsible for the generation of electricity and magnetism. Metals have free electrons that can move from one atom to the other within the metal. In fact, this factor is responsible for the excellent electrical conductivity. But if they try to escape the metal surface, they are unable to do so. This is because when these negatively charged particles (electrons) try to leave the metal, the surface of the metal acquires a positive charge. Due to the attraction between the negative and the positive charges, the electrons are pulled back into the metal. There exist no forces to pull them forward. The electrons are thus forced to stay inside the metal due to the attractive forces. This barrier provided by the metal surface to prevent escaping of free electrons is called the surface barrier.
However, the surface barrier can be broken by providing a certain minimum amount of energy to the free electrons which increases their kinetic energy and consequently help them escape the metal surface. This minimum amount of energy is known as the work function of the metal. And when the work function is provided to the metal, the consequent liberation of electrons from the metal surface is known as electron emission.
The work function of a metal depends on:
- The properties of the metal
- The purity of the metal
- The nature of the metal surface
Types of Electron Emission
The electron emission is possible only if sufficient energy (equal to the work function of the metal) is supplied to the metal in the form of heat energy, light energy, etc. Depending on the source of energy, electron emission can be of the following types:
- Thermionic Emission: In this type, the metal is heated to a sufficient temperature to enable the free electrons to come out of its surface.
- Field Emission: In this type, a very strong electric field is applied to the metal which pulls the electrons out of the surface due to the attraction of the positive field.
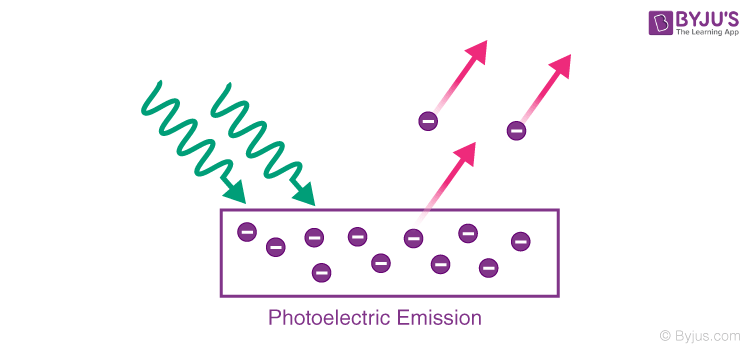
- Photoelectric Emission: In this type, the light of a certain frequency is made to fall on the metal surface which leads to the emission of electrons.
Frequently Asked Questions – FAQs
What is work function?
What are free electrons?
What is field emission?
What is cathode ray tube?
In photoelectric emission, which form of energy is supplied to the electrons to escape the metal?
Stay tuned with BYJU’S to learn more about electron emission and other concepts in quantum mechanics with interesting video lectures. Also, register to “BYJU’S – The Learning App” for loads of interactive, engaging Physics-related videos and unlimited academic assistance.
Hertz and Lenard’s Observation of Photoelectric Effect

Comments