Potassium Acetate formula, also known as Potassium salt formula or Diuretic salt formula is explained in this article. It is an essential macromineral and is the acetate salt form of potassium. It consists of an equal number of acetate and potassium ions. The molecular or chemical formula of Potassium Acetate is C2H3KO2.
Potassium Acetate occurs either as a white crystalline powder or as colourless deliquescent crystals. It has a faint acetic smell. It is soluble in water, alcohol, liquid ammonia, methanol, ethanol. It is insoluble in acetone and ether. It is widely used as a deicer to remove ice as well as to prevent its formation.
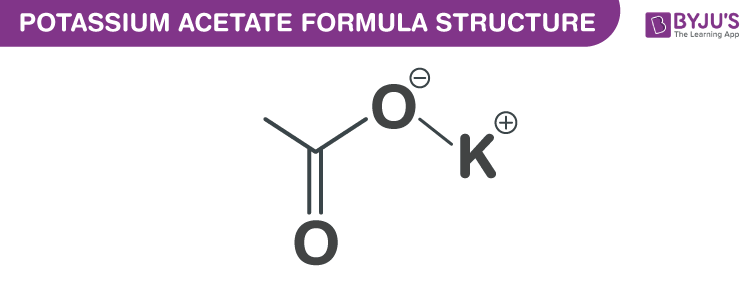
Potassium Acetate Formula Structure
Properties Of Potassium Acetate Formula
| Chemical formula | C2H3KO2 |
| Molecular weight | 98.142 g/mol |
| Density | 1.8 g/cm3 (20 °C)
1.57 g/cm3 at (25 °C) |
| Boiling point | Decomposes |
| Melting point | 292 °C |
Potassium salt can be prepared by acid-base neutralization reaction. Treat potassium carbonate or potassium hydroxide (potassium-containing base) with acetic acid to obtain potassium acetate.
To learn more about Potassium acetate formula from the expert faculties at BYJU’S, register now!
Comments