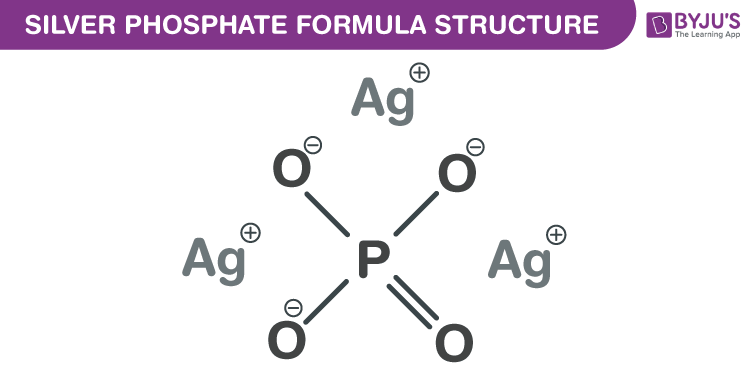
Silver phosphate formula, also known as Trisilver phosphate formula or Silver orthophosphate formula is discussed in this article. The structure of this inorganic salt is formed by one phosphate anion and three silver cations. The molecular or chemical formula of Silver phosphate is Ag3PO4.
Silver orthophosphate is yellow solid in its pure form and turns darker as it becomes impure. It is insoluble in water but soluble in solutions and acids. It is synthesized by reacting the salt orthophosphate and silver nitrate. The product got is insoluble in water. The yellow precipitate is filtered to recover as a pure solid.
Silver phosphate Formula Structure
Properties Of Silver phosphate Formula
| Chemical formula | Ag3PO4 |
| Molecular weight | 418.574 g/mol |
| Density | 6.370 g/cm3 |
| Appears as | Yellow solid |
| Melting point | 849 °C |
This compound causes coughing and respiratory disease. It also affects skin and eyes and can cause serious damage to eyes.
To learn more about Silver phosphate formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Trisilver phosphate for free.
Comments