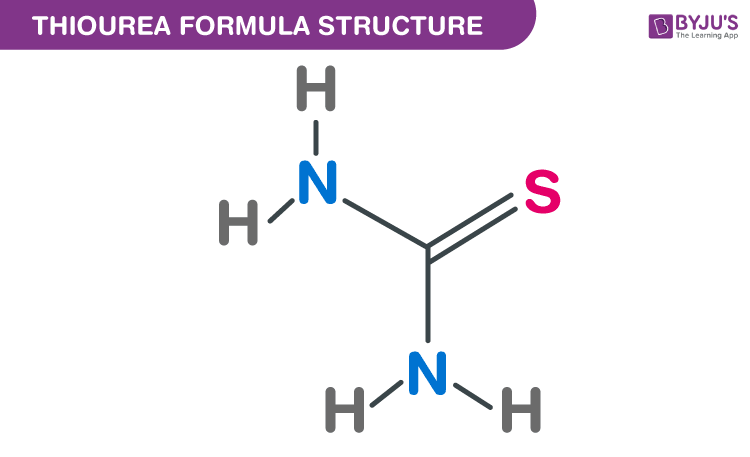
Thiourea formula, also named as Pseudothiourea formula or Thiocarbamide formula is discussed in this article. It is an organosulfur compound and its structure is similar to urea, except that the sulfur atom replaces the oxygen atom. The molecular or chemical formula of Thiourea is CH4N2S.
Thiocarbamide is a crystalline solid white in colour. It is found naturally and also can be synthesized. It dissolves in water, ethanol, and ammonium thiocyanate solution.
Thiourea Formula Structure
Properties Of Thiourea Formula
| Chemical formula | CH4N2S |
| Molecular weight | 76.12 g/mol |
| Density | 1.405 g/ml |
| Appearance | white solid |
| Melting point | 182 °C |
Use Of Thiourea
- It is widely used as silver tarnish removers and in animal glue liquefiers.
- In a textile industry thiourea is a common reducing agent.
- It is used in the production of vulcanization accelerator and flame retardent resign.
Safety Measures
- When this compound is heated and decomposes it emits toxic fumes of sulfur oxides and nitrogen oxides.
- Prolonged exposure to this compound can cause bone marrow damage in humans.
To learn more about Thiourea formula from the expert faculties at BYJU’S, register now! Also, you can download notes on Thiocarbamide for free.
Comments