Reverse Osmosis Definition
“Reverse osmosis is a special type of filtration that uses a semi-permeable, porous membrane, that allows only pure water to pass through it, filtering the larger molecules or impurities.”
What is Reverse Osmosis?
Reverse osmosis is the process in which pressure is applied to overcome colligative property and osmotic pressure that is directed by a thermodynamic parameter and a chemical difference of a solvent.
This application is mainly applied in the production of potable water in water plants and in industries. The end result will be the solute. It happens when the pure solvent is allowed to follow to one end of the membrane thus allowing a solute to retain in a permissible side of a membrane. Reverse osmosis removes suspended and types of dissolved species from water including bacteria.
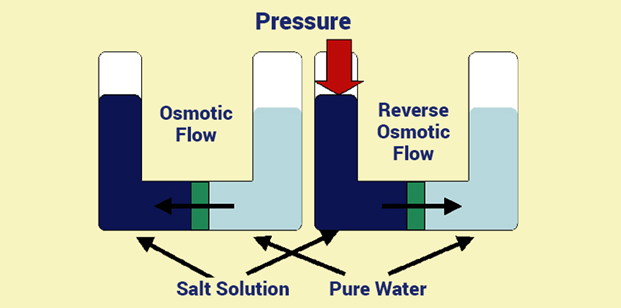
Reverse Osmosis Principle
Reverse osmosis works by reversing the principle of osmosis. The salt solution is subjected to pressure and pressed against the semi-permeable membrane. Here, the applied pressure is greater than the osmotic pressure. Thus, the molecules move from a highly concentrated solution to a less concentrated solution.
Also Read: Osmosis
Working of Reverse Osmosis
Diffusion is a process by which the molecules move from the region of higher concentration to lower concentration. There is a net movement meaning more molecules moving in one direction than in the opposite direction.
In osmosis, the water molecules and the concentration gradient occurs over the semipermeable membrane which allows the entry of water and blocks the passage of ions and other larger molecules including sodium, chlorine, bacteria, glucose, etc.
Reverse osmosis is the process or the technology which is used to remove ions, mineral chemicals, and other impurities from drinking water. In this process, greater pressure is applied, forcing the water to travel through the semipermeable membrane in opposite to natural osmosis.
Reverse Osmosis works on the same principle as osmosis, but in the reverse direction. In this process direction of water flow is reversed by applying greater pressure.
For instance, consider a semipermeable membrane placed between the freshwater and concentrated aqueous solution. In natural osmosis, the freshwater will cross the semipermeable membrane and dilutes the concentrated solution. In reverse osmosis, the pressure is applied towards the concentrated aqueous solution and the water molecules are forced to cross the membrane towards the freshwater.
Contaminants Removed by Reverse Osmosis from Water
Reverse osmosis removes 99% of dissolved salts particles, colloids, bacteria, pyrogens from feed water. The contaminants are separated by the RO membrane on the basis of size and charge. The smaller the charge of the contaminant, the more are the chances for it to pass through the RO membrane. For eg., sodium and calcium are monovalent and divalent respectively. Due to their smaller charges, they can easily pass through the membrane. Similarly, RO cannot remove gases such as carbon dioxide from the water because they are not highly ionized.
Difference between Osmosis and Reverse Osmosis
Following are the major differences between osmosis and reverse osmosis:
|
Osmosis |
Reverse Osmosis |
|
This is the process by which the molecules of a solvent pass through the semi-permeable membrane from a region of lower concentration to a higher concentration. |
This is the process by which the molecules of a solvent pass through the semi-permeable from a region of higher concentration to lower concentration when pressure greater than the osmotic pressure is applied. |
| It is a natural process. | It is an artificial process. |
| Occurs along the potential gradient. | Occurs against the potential gradient. |
| This is observed during the opening of stomata and absorption of water from the soil by the roots. | This is used in water purification systems. |
Also Read: Difference between Osmosis and Diffusion
To know more about what is reverse osmosis and how does it function, keep visiting BYJU’S website or download BYJU’S app for further reference.

good narration with examples