Osmosis Definition
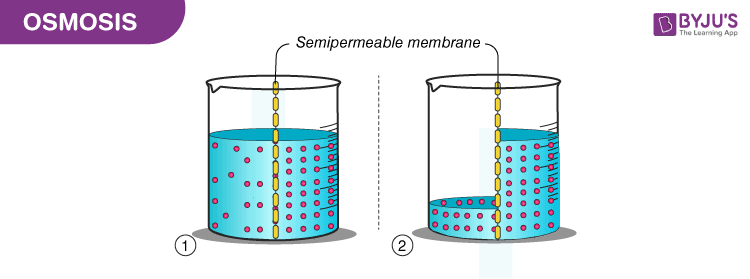
“Osmosis is a process by which the molecules of a solvent pass from a solution of low concentration to a solution of high concentration through a semi-permeable membrane.”
Osmosis
Table of Contents

What is Osmosis?
Osmosis is a passive process and happens without any expenditure of energy. It involves the movement of molecules from a region of higher concentration to lower concentration until the concentrations become equal on either side of the membrane.

Any solvent can undergo the process of osmosis including gases and supercritical liquids.
Let us have a detailed look at the different types and effects of osmosis in detail.
Also Refer: Difference between osmosis and diffusion
Osmotic Solutions
There are three different types of solutions:
-
Isotonic Solution
-
Hypertonic Solution
-
Hypotonic Solution
An isotonic solution is one that has the same concentration of solutes both inside and outside the cell.
A hypertonic solution is one that has a higher solute concentration outside the cell than inside.
A hypotonic solution is one that has a higher solute concentration inside the cell than outside.
Types of Osmosis
Osmosis is of two types:
-
Endosmosis– When a substance is placed in a hypotonic solution, the solvent molecules move inside the cell and the cell becomes turgid or undergoes deplasmolysis. This is known as endosmosis.
-
Exosmosis– When a substance is placed in a hypertonic solution, the solvent molecules move outside the cell and the cell becomes flaccid or undergoes plasmolysis. This is known as exosmosis.
Also Read: Difference between endosmosis and exosmosis
Effect of Osmosis on Cells
Osmosis affects the cells differently. An animal cell will lyse when placed in a hypotonic solution compared to a plant cell. The plant cell has thick walls and requires more water. The cells will not burst when placed in a hypotonic solution. In fact, a hypotonic solution is ideal for a plant cell.
An animal cell survives only in an isotonic solution. In an isotonic solution, the plant cells are no longer turgid and the leaves of the plant droop.
The osmotic flow can be stopped or reversed, also called reverse osmosis, by exerting an external pressure to the sides of the solute. The minimum pressure required to stop the solvent transfer is called the osmotic pressure.
Osmotic Pressure

Osmotic pressure is the pressure required to stop water from diffusing through a membrane by osmosis. It is determined by the concentration of the solute. Water diffuses into the area of higher concentration from the area of lower concentration. When the concentration of the substances in the two areas in contact is different, the substances will diffuse until the concentration is uniform throughout.
Osmotic pressure can be calculated using the equation:
Π=MRT
where Π denotes the osmotic pressure,
M is the molar concentration of the solute,
R is the gas constant,
T is the temperature
Significance of Osmosis
- Osmosis influences the transport of nutrients and the release of metabolic waste products.
-
It is responsible for the absorption of water from the soil and conducting it to the upper parts of the plant through the xylem.
-
It stabilizes the internal environment of a living organism by maintaining the balance between water and intercellular fluid levels.
-
It maintains the turgidity of cells.
-
It is a process by which plants maintain their water content despite the constant water loss due to transpiration.
-
This process controls the cell to cell diffusion of water.
-
Osmosis induces cell turgor which regulates the movement of plants and plant parts.
-
Osmosis also controls the dehiscence of fruits and sporangia.
-
Higher osmotic pressure protects the plants against drought injury.
Also Refer: Passive Transport
Examples of Osmosis
Osmosis has a significant role to play in plants, animals and also in humans. In an animal cell, osmosis helps in absorbing water from the intestines to the blood.
Listed below are more examples of Osmosis.
-
The absorption of water from the soil is due to osmosis. The plant roots have a higher concentration than the soil. Therefore, the water flows into the roots.
-
The guard cells of the plants are also affected by osmosis. When the plant cells are filled with water, the guard cells swell up, and the stomata open.
-
If a freshwater or saltwater fish is placed in the water with different salt concentrations, the fish dies due to the entry or exit of water in the cells of the fish.
-
Humans suffering from cholera are also affected by osmosis. The bacteria that overpopulate the intestines reverse the flow of absorption and do not allow water to be absorbed by the intestines, which results in dehydration.
-
When the fingers are placed in water for a longer period of time, they become pruney due to the flow of water inside the cells.
Also Read: Reverse Osmosis
For more information on osmosis, its definition, types, effects and osmotic pressure, keep visiting BYJU’S Biology website or download the BYJU’S app for further reference.
Frequently Asked Questions
How do you define osmosis?
Osmosis is the movement of solvent from a region of lower solute concentration to a region of higher solute concentration through a semi-permeable membrane.
What are the three types of osmotic conditions that affect living cells?
The three types of osmotic conditions include- hypertonic, isotonic, and hypotonic.
What are the different types of osmosis?
The different types of osmosis include:
- Endosmosis- when a substance is placed in a hypotonic solution, the solvent molecules move inside the cell and the cell becomes rigid.
- Exosmosis-when a substance is placed in a hypertonic solution, the solvent molecules move out of the cell and the cell becomes flaccid.
Why is osmosis important for the cells?
Osmosis is important for the cells for many reasons. It helps in the movement of important materials inside and out of the cell. The nutrients, water and other solutes move in and out of the cell by the process of osmosis.
How is osmosis different from diffusion?
Osmosis is a process of movement of solvents through a semi-permeable membrane from a region of lower solute concentration to higher solute concentration. On the contrary, diffusion does not require a semi-permeable membrane to occur and the molecules move from a region of higher concentration to lower concentration.
Do dead cells exhibit osmosis?
What is the main function of osmosis?
Osmosis helps in stabilizing the internal environment of the organism by balancing the levels of water and intracellular fluids. Also, the nutrients and minerals enter the cell by osmosis which is necessary for the survival of cells.
What is forward osmosis?
Forward osmosis is a natural phenomenon that occurs around us on a daily basis. It is the type of osmosis that uses a semi-permeable membrane in the separation of water from dissolved solutes. This type of osmosis is widely used in wastewater treatment, osmotic power generation, etc.
List of some examples of osmosis.
The real-life examples of osmosis are:
- Feeling thirsty after having salty food.
- Dialysis of kidney in the excretory system.
- Swelling of resins and other seeds when they are soaked in water.
- Movement of salt-water in the animal cell across our cell membrane.
- Movement of water and minerals from root nodules to various parts of plants.
What are the factors affecting the rate of Osmosis?
The factors affecting the rate of osmosis include:
- Pressure.
- Temperature.
- Surface Area.
- Water Potential.
- Concentration gradient.
What is Osmotic pressure?
Osmotic pressure is defined as the minimum pressure applied to a solution to stop the flow of solvent molecules through a semipermeable membrane. The osmotic pressure of a solution is proportional to the molar concentration of the solute particles in the solution.
π = iCRT is the formula used for finding the osmotic pressure of a given solution.
What is a semipermeable membrane?
The semipermeable membrane is a biological membrane, which functions by permitting the movements of certain molecules or ions to pass through it.
What is reverse osmosis?
Reverse osmosis is a natural phenomenon that occurs in the opposite direction of the natural osmosis. This type of osmosis is used for removing the majority of contaminants from water by pushing the water under pressure through a semi-permeable membrane.
What is the significance of osmosis?
The biological importance of osmosis includes:
- It is essential for the survival of a cell.
- Osmosis plays a key role during the germination of seeds.
- Involved in the movement of water molecules between the cell and cell organelles.
- In plants, it is involved in the movement of water molecules from the soil into the root nodules.
- The mechanism of stomata is mainly because of the response to the osmotic pressure of the guard cells in relation to the epidermal cells.


Wonderful article up here.
Really helpful
This page very important for me found for some answer
Very helpful
Awesome
Really Helpful. Thanks.
Osmosis and Reverse Osmosis are well explained.
Very useful for students and Teachers. Keep covering more topics.
Really helpful and meaningful.
Thanks BYJU’S
useful
So good it really helps my report thanks 🙂
I like this
I like this page
thank you
Wonderful app Byju’s 😄👍