Table of Contents
- What is Diffusion?
- Types of Diffusion
- Factors affecting Diffusion
- Examples of Diffusion
- Causes of Diffusion
- Significance of Diffusion
Diffusion Definition
“Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration down the concentration gradient.”
Read on to explore what is diffusion and the different types of diffusion.
What is Diffusion?
Diffusion is the process of movement of molecules under a concentration gradient. It is an important process occurring in all living beings. Diffusion helps in the movement of substances in and out of the cells. The molecules move from a region of higher concentration to a region of lower concentration until the concentration becomes equal throughout.
Liquid and gases undergo diffusion as the molecules are able to move randomly.
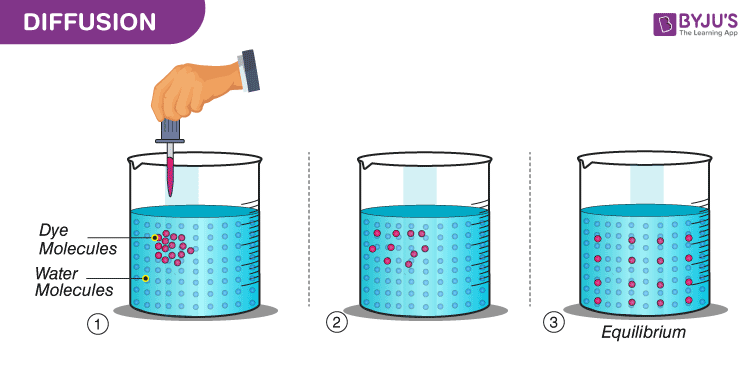
Example:
Take water in a beaker. Add a few copper sulfate crystals in one place and leave it as it is for some time without disturbing it. After some time we can see that the beaker contains a uniformly coloured solution. Here, both water and copper sulfate diffuse independently. With this experiment, we can infer that solutes move from a higher concentration to a lower concentration in a solution.
Also Read: Diffusion in Plants
Recommended Video:
Types of Diffusion
Diffusion is widely used in various fields such as biology, physics, chemistry, etc. Diffusion can be classified into two main types: Simple diffusion and facilitated diffusion.
Simple diffusion
A process in which the substance moves through a semipermeable membrane or in a solution without any help from transport proteins. For example, bacteria deliver small nutrients, water and oxygen into the cytoplasm through simple diffusion.
Facilitated diffusion
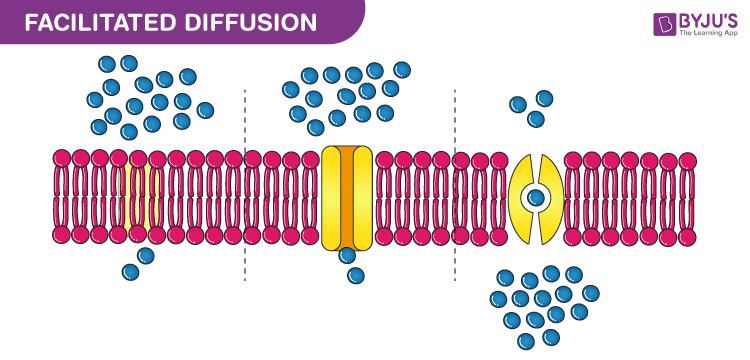
Facilitated diffusion is a passive movement of molecules across the cell membrane from the region of higher concentration to the region of lower concentration by means of a carrier molecule.
Dialysis: It is the diffusion of solutes across a selectively permeable membrane. A selectively permeable membrane is one that allows only specific ions and molecules to pass through, while it obstructs the movement of others.
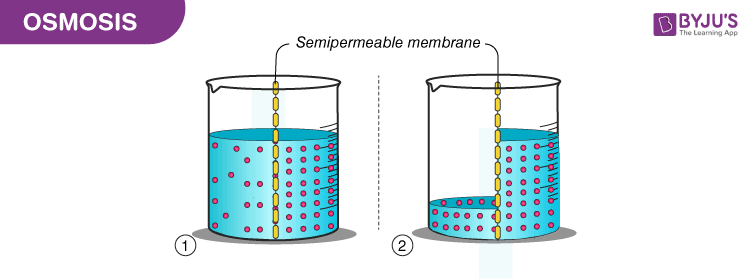
Osmosis: It is the movement of solvent molecules from the region of lower concentration to the region of higher concentration through a semipermeable membrane. Since water is solvent in every living being, biologists define osmosis as the diffusion of water across a selectively permeable membrane. For example, plants take water and minerals from roots with the help of osmosis.
Also Read: Facilitated Diffusion
Factors affecting Diffusion
There are a few factors that affect the process of diffusion, which individually and collectively alters the rate and extent of diffusion. These factors include:
- Temperature.
- Area of Interaction.
- Size of the Particle.
- The steepness of the concentration gradient.
Examples of Diffusion
- A tea bag immersed in a cup of hot water will diffuse into the water and change its colour.
- A spray of perfume or room freshener will get diffused into the air by which we can sense the odour.
- Sugar gets dissolved evenly and sweetens the water without having to stir it.
- As we light the incense stick, its smoke gets diffused into the air and spreads throughout the room.
- By adding boiling water to the dried noodles, the water diffuses causing rehydration and making dried noodles plumper and saturated.
Causes of Diffusion
Diffusion is a natural and physical process, which happens on its own, without stirring or shaking the solutions. Liquid and gases undergo diffusion as the molecules are able to move randomly. The molecules collide with each other and change their direction.
Significance of Diffusion
Diffusion is an important process, which is involved in the different life processes. As mentioned above, it is the net movement of particles, ions, molecules, solution, etc. In all living species, diffusion plays an important role in the movement of the molecules during the metabolic process in the cells.
Diffusion is important for the following reasons:
- During the process of respiration, this process helps in diffusing the carbon dioxide gas out through the cell membrane into the blood.
- Diffusion also occurs in plant cells. In all green plants, water present in the soil diffuses into plants through their root hair cells.
- The movement of ions across the neurons that generates electrical charge is due to diffusion.
Also Read: Difference between diffusion and osmosis
Frequently Asked Questions
1. What is diffusion?
Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration down the concentration gradient.
2. List the types of diffusion.
Diffusion can be divided into two main types, namely, simple diffusion and facilitated diffusion.
3. What is simple diffusion?
Simple diffusion is defined as the process in which a substance moves through a semipermeable membrane or in a solution without any help from transport proteins.
4. State an example of simple diffusion.
In a cell, water, oxygen and carbon dioxide molecules can pass directly through the cell membrane without requiring any energy along the concentration gradient. This is a form of simple diffusion.
5. What is facilitated diffusion?
Facilitated diffusion can be defined as the passive movement of molecules across the cell membrane from a region of higher concentration to a region of lower concentration by means of a carrier molecule.
6. Provide an example of facilitated diffusion.
In the human body, glucose molecules, sodium and potassium ions use carrier proteins to pass through the cell membranes.
7. How does dialysis work?
Dialysis works through the diffusion of solutes across a selectively permeable membrane. A selectively permeable membrane is the one that allows only specific ions and molecules to pass through while obstructing the movement of other molecules.
8. What are the factors affecting diffusion?
Temperature, area of interaction, size of the particle and the steepness of the concentration gradient are all factors that affect the process of diffusion.
9. State the significance of diffusion.
Diffusion is a very important process occurring in all living beings. All living organisms exhibit one or the other form of diffusion, allowing the movement of the molecules during various metabolic or cellular processes.
Learn more about diffusion, its definition, types examples, and other related topics at BYJU’S Biology

It very much good and helpful 😊
VERY MUCH HELPFUL
This was rellay helpful now i can easily do my upcoming exams
Thanks alot
This is very helpful. Thank u team Byjus.
😊 very good and helpful
😊 very good and helpful
Thanks this was very helpful
Thanks it is so helping
What is the purpose of diffusion?
Diffusion is the way of passive transport of substances in and out of the cell across the cell membrane. Diffusion occurs down the concentration gradient, i.e. from higher concentration to lower concentration.
Helpful