What is an Acid Anhydride?
An acid anhydride is a molecule that is capable of forming an acidic solution in water. Before going deep into it. Let’s remember some basic concepts.
- Acids – These are the substances that are ready to donate hydrogen ions in water.
- Bases – these are the substances that hydroxide ions in water.
Table of contents
- What Is Anhydride?
- Definition Of Acid Anhydride
- Synthesis Of Acid Anhydride
- Chemical Properties Of Acid Anhydride
- Uses Of Acid Anhydride
- FAQs
What is Anhydride?
Anhydride literally means ‘without water. It can be defined as the chemical compound formed by eliminating water from another compound. Anhydride reacts with water to produce either base or an acid.
Acid Anhydrides – Definition & Meaning
An Acid anhydride can be defined as a non-metal oxide which forms an acidic solution when reacted with water. In organic chemistry, it is a functional group consisting of 2 acyl groups combined by an oxygen atom. The non-metals which are capable of reacting with water are only called Acid anhydrides and non-metals that do not react with water are not acids anhydrides.
So we can conclude that all non-metals are not acid anhydride; those non-metals that react with water from acid are only acid anhydride. For example, carbon monoxide is not an acid anhydride, though it is an oxide of carbon because it does not react with water.
In the case of organic chemistry, acid anhydrides are formed from the dehydration of two carboxylic acid groups.
Let’s try to understand better with the following reactions
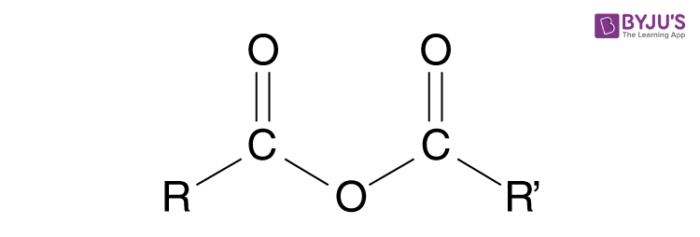
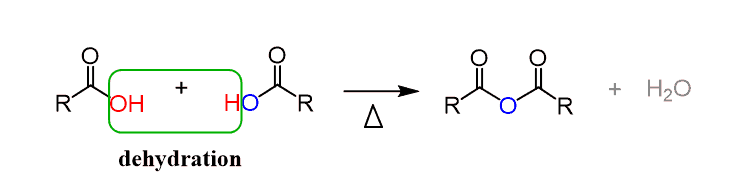
Synthesis of Acid Anhydride
- An organic acid anhydride is synthesized by the heating of two carboxylic acids at a high temperature of nearly 800℃. In this process, one water molecule is removed from the reaction. It can be synthesized by the reaction of a carboxylic acid with P2O5.
Chemical properties of acid
- Acid anhydride undergoes a nucleophilic substitution reaction by substituting its -OCOR group. It is less reactive than acid chlorides because Cl atom is more electronegative compared to -OCOR group.
 Formation of Carbonic acid
Formation of Carbonic acid
- When carbon dioxide reacts with water, it forms carbonic acid. The chemical equation will be like this. CO2(g) + H2O → H2CO3(aq)
- As discussed earlier, Carbon Dioxide is a non-metal reacts with water to form H2CO3 (Carbonic acid) which is acid as it has hydrogen to donate. This reaction is responsible for acid rain. It also plays a key role in changing the pH levels of streams, rivers and oceans. It can be observed in the above equation that if there is more CO2 in the air, more carbonic acid is produced which is harmful to life. This is the major problem we are facing today.
Formation of Sulphuric acid:
- When Sulphur trioxide reacts with water it forms Sulphuric acid. It can be chemically explained like this.
SO3(g) + H2O → H2SO4(aq)
- Later, Sulphur trioxide gas reacts with water and resulted in the formation of Sulphuric acid. Sulphur dioxide in the air reacts with oxygen and forms sulphur trioxide. When it reacts with water during rain, that is how acid rain occurs. These have very harmful effects on the environment.
Uses of Acids Anhydrides
Acid anhydrides have wide uses in organic chemistry.
- They are used in the manufacture of many things like pharmaceuticals, industrial chemicals, explosives and perfumes.
- Synthesis of esters by acetylation of alcohols
- Synthesis of aspirin (Acetylsalicylic acid)
- Synthesis of heroin by deacetylation of morphine
- Use as a protecting group
Frequently Asked Questions – FAQs
Why are acid anhydrides so reactive?
Anhydrides are less stable because the electron donation to one carbonyl group competes with the electron donation to the second carbonyl group. Thus, compared with esters, where only one carbonyl group has to balance the oxygen atom, anhydrides are more reactive than esters.
Are acid anhydrides always symmetrical?
Acid anhydrides are derivatives of carboxylic acids, as the name suggests. They may be symmetric in theory (where the two groups of R are identical) or asymmetric (where the two groups of R are different). Di carboxylic acid-derived cyclic anhydrides are known as -di-oic anhydrides.
Are acid anhydrides reactive?
Acid anhydrides are formed from the dehydration reaction of two classes of carboxylic acids, as their name suggests. Anhydrides are highly reactive to nucleophiles and are capable of acylation a variety of proteins and other macromolecules that are important functional groups.
Are amides more reactive than ketones?
Amides can also be hydrolysed in an acidic state. In this case, amide O is first protonated, followed by water’s nucleophilic attack on carbon. There is no amide-like resonance in the case of ketone, and a nucleophile will strike its C of C=O. It’s relatively easier.
How acid anhydrides are formed?
A compound that has two acyl groups (R-C=O) bound to the same oxygen atom is an acid anhydride. Anhydrides are typically formed in the presence of a base when carboxylic acid reacts with acid chloride.



Nice app