What is Alcohol in Chemistry?
Alcohol is an organic compound with at least one hydroxyl functional group (OH) bound to a saturated carbon atom, according to chemistry.
Table of Contents
- Types of Alcohols Primary Secondary and Tertiary Alcohols
- Recommended Videos
- Oxidation of Alcohols
- Dehydration of Alcohols
- Esterification of Alcohols
- Frequently Asked Questions – FAQs
Alcohol is a homologous sequence of compounds that contain the hydroxyl group as a functional group (-OH). Alcohols have the general molecular formula CnH2n+1OH. Alcohols are hydrocarbon compounds with one or two hydroxyl groups replacing one or more hydrogen atoms in the hydrocarbon.
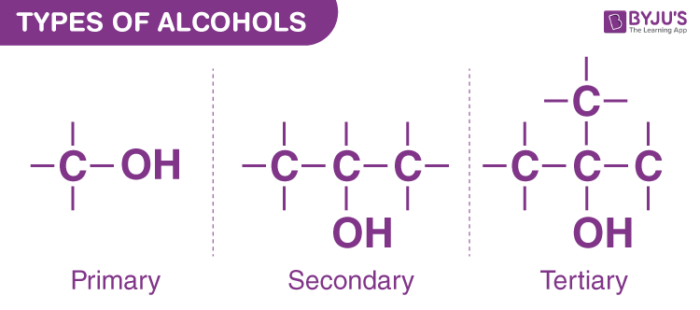
Types of Alcohols – Primary, Secondary and Tertiary Alcohols
Primary, secondary, and tertiary alcohols are the three types of alcohols. Alcohols are divided into three categories: primary, secondary, and tertiary. The hydroxyl group is classified according to where an alkyl group’s carbon atom is bound to the hydroxyl group.
In chemistry, an alcohol is formed when the hydrogen atom in a hydrocarbon is replaced by a hydroxyl group, which is made up of two oxygen and hydrogen atoms. Secondary alcohols are formed when alcohols interact with other atoms.
Recommended Videos

The Alcohol Reaction Map
Alcohol Reaction Alcohols are sometimes referred to as the core functional group because they are the source of all other functional groups. Here’s a list of some way’s alcohol can be transformed.
Oxidation of Alcohols
In organic chemistry, the oxidation of alcohol is a crucial reaction. Aldehydes and carboxylic acids are formed when primary alcohols are oxidised; ketones are formed when secondary alcohols are oxidised. Tertiary alcohols, on the other hand, cannot be oxidised without breaking the C–C bonds in the molecule.
1. Oxidation of Primary Alcohols
Carboxylic Acids are made by oxidising primary alcohols or aldehydes. In the presence of dilute sulphuric acid, primary alcohols and aldehydes are usually oxidised to carboxylic acids using potassium dichromate(VI) solution. The potassium dichromate(VI) solution changes colour from orange to green during the reaction.
2. Oxidation of Secondary Alcohols
As a secondary alcohol is oxidised, it becomes a ketone. Along with the hydrogen bound to the second carbon, the hydrogen from the hydroxyl group is lost. The oxygen that is left forms double bonds with the carbon. As R1–COR2, a ketone is formed. Secondary alcohols are readily oxidised up to the ketone level without breaking carbon-carbon bonds. Except in extreme conditions, no further oxidation occurs.
For example, the oxidation reaction of propan-2-ol (secondary alcohol) which is oxidised to propanone (ketone) is given below.
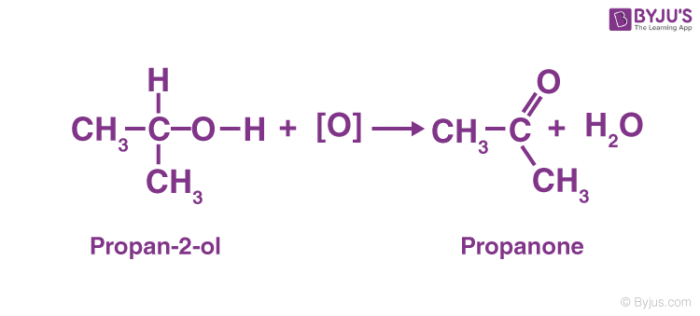
3. Oxidation of Tertiary Alcohols
Since the carbon atom that holds the OH group does not have a hydrogen atom attached to it and is instead bound to other carbon atoms, tertiary alcohols (R3COH) are immune to oxidation. Under oxidative conditions, carbon-to-hydrogen bonds are easily broken, but carbon-to-carbon bonds are not.
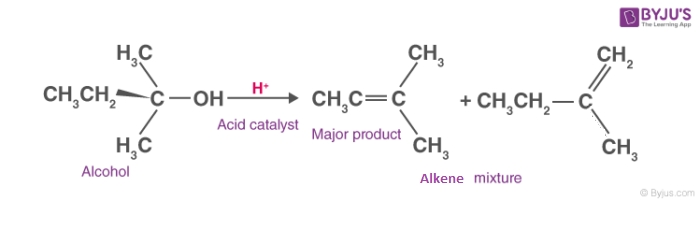
Dehydration of Alcohols
Dehydrogenation is an important process in petroleum chemistry because it converts inert alkanes into olefins and aromatic compounds, which serve as starting points for other functional groups.
Alcohol dehydration generally requires the cleavage of a C-O bond and the loss of a proton from the beta place. The formation of a chemical known as the carbo cation intermediate occurs during the dehydration of secondary and tertiary alcohols.
The following is a depiction of the general dehydration reaction of alcohols:
Esterification of Alcohols
Esterification is a chemical reaction in which two reactants (usually an alcohol and an acid) combine to produce an ester as the reaction product. Esters are widely used in organic chemistry and biological materials, and they also have a good fruity odour.
Esters are formed when alcohols react with a variety of acids. Fischer’s esterification is characterized by the reaction of an alcohol with an acid (catalysed by acid) to produce an ester plus water. Inorganic acids may also react with alcohols to form esters under the right conditions.
To produce a small ester, such as ethyl ethanoate, gently heat a mixture of ethanoic acid and ethanol in the presence of concentrated sulphuric acid, then distil off the ester as soon as it forms. This prevents the opposite reaction from occurring.
Esterification, a reaction in which a carboxylic acid and an alcohol are heated in the presence of a mineral acid catalyst to form an ester and water, may be used to make certain esters: The reaction can be reversed. Butyl acetate can be made from acetic acid and 1-butanol as an example of an esterification reaction.
Frequently Asked Questions – FAQs
What are alcohols in organic chemistry?
Alcohols are a chemical family that includes compounds with one or more hydroxyl (-OH) groups bound to a single bonded alkane. The general formula -OH is used to describe alcohols. Alcohols are useful in organic chemistry because they can be converted into and out of a variety of other compounds.
Is 1 Pentanol a primary alcohol?
1-Pentanol, also known as butyl carbinol or 1-pentyl alcohol, belongs to the main alcohol class of organic compounds. Primary alcohols have the general structure RCOH and contain the primary alcohol functional group.
What is the order of esterification?
Alcohols are esterified in the following order: 1>2>3. The order of esterification decreases as the steric hindrance (or bulkiness) increases from primary to secondary to tertiary alcohol.
Are carboxylic acids Electrophiles?
Carboxylic acids are not especially electrophilic, and most nucleophiles avoid them. Acid chlorides, on the other hand, are extremely violent electrophiles that readily react with weak nucleophiles such as water or alcohols.
Can two alcohols react?
It is possible for two alcohol molecules to dehydrate each other under the right conditions. The hydrogen atom of one molecule’s OH group is removed, while only the hydrogen atom of the second molecule’s OH group is removed. An ether molecule is made up of two ethyl groups bound to an oxygen atom.
Do secondary alcohols react with sodium?
When ethanol reacts with sodium metal (a base), sodium ethoxide and hydrogen gas are produced. This reaction is the same as when sodium metal reacts with water. Similarly, potassium metal undergoes similar reactions. If you progress from primary to secondary to tertiary, the acidity of alcohol decreases.


Comments