What are Aromatic Compounds?
Aromatic compounds are chemical compounds that consist of conjugated planar ring systems accompanied by delocalized pi-electron clouds in place of individual alternating double and single bonds.
They are also called aromatics or arenes. The best examples are toluene and benzene. Aromatics require satisfying Huckel’s rule. Plants and micro-organisms have an exclusive route to benzene-ring compounds. The great majority of aromatic compounds in nature, therefore, are produced by plants and micro-organisms, and animals are dependant upon plants for many aromatic compounds either directly or indirectly.
Table of Contents
- Recommended Videos
- Aromatic Compounds Examples
- Properties of Aromatic Compounds
- IUPAC Nomenclature of Aromatic Compounds
- Frequently Asked Questions – FAQs
Recommended Videos
Aromatic Compounds Examples
Aromatic hydrocarbon, are hydrocarbons containing sigma bonds and delocalized pi electrons between carbon atoms in a ring. For example, benzene. They are known as aromatic due to their pleasant smell.
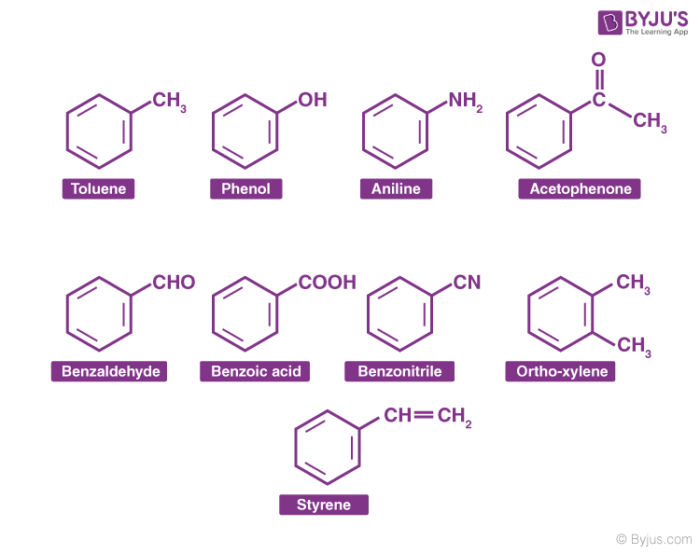
Aromatic compounds are broadly divided into two categories: benzenoids (one containing benzene ring) and non-benzenoids (those not containing a benzene ring) for example, furan. Any hydrocarbon can be classified as an aromatic compound provided they follow the Huckel rule. According to Huckel rule, for a ring to be aromatic it should have the following properties:
- Planarity
- Complete delocalization of the π electrons in the ring
- Presence of (4n + 2) π electrons in the ring where n is an integer (n = 0, 1, 2, . . .)
Huckel’s Rule of Aromaticity
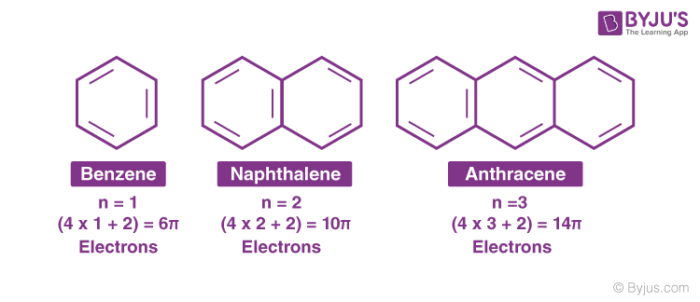
Huckel’s rule states that only planar, fully conjugated monocyclic polyenes having 4n + 2 π electrons, where n is an integer, that is, n = 0, 1, 2, 3, 4, etc., should possess aromatic stability. An aromatic compound must be planar and contain a cyclic cloud of π electrons below and above the plane of the molecule. It contains sp2 hybridized carbon atoms and must obey the Huckel rule.
According to this rule, the ring system must have (4n+2) π electrons, where n is any whole number (0, 1, 2, 3, etc). On this basis the ring systems which have 2(n=0), 6(n=1), 10(n=2), 14(n=3) etc pi electrons are aromatic. Typical examples of aromatic compounds are benzene, naphthalene, and anthracene.
Properties of Aromatic Compounds
Arenes are mostly nonpolar and non-miscible in water. These compounds are usually unreactive and are used as solvents for various other nonpolar compounds. Their carbon to hydrogen ratio is high therefore, they are characterized by sooty yellow flame.
Classification of Aromatic Compounds
The classification of arenes is based on the position of the functional group. They are classified into two and we have discussed below:
1. Nuclear Substituted Compounds
In any aromatic compound whenever any substituent or functional group, is directly linked to the benzene ring, it is known as a nuclear-substituted compound.
2. Side chain Substituted Compounds
In any aromatic compound if the functional group is available in the side chain of the ring then it is known as a side chain substituted compound. These compounds are named as the phenyl derivatives of the relative aliphatic compounds.
IUPAC Nomenclature of Aromatic Compounds
Earlier, most of the compounds with the same structural formula were known by different names depending on the regions where they were synthesized. This naming system was very trivial since it raised a lot of confusion. Finally, a common naming system enlisting standard rules was set up by IUPAC (International Union for Pure and Applied Chemistry) for the naming of compounds. This method of naming is IUPAC naming or IUPAC nomenclature.
IUPAC nomenclature of aromatic hydrocarbons is explained below:
1. According to IUPAC nomenclature of substituted aromatic compounds, the substituent name is placed as a prefix to the name of aromatic compounds. For example, a benzene ring attached to a one-nitro group is named as nitrobenzene.

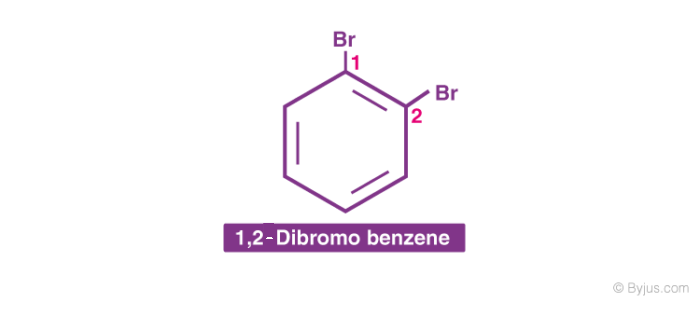
2. When more than one similar substituent group is present in the ring, they are labelled with the Greek numerical prefixes such as di, tri, tetra to denote the number of similar substituent groups attached to the ring. If two bromo- groups are attached to the adjacent carbon atoms of the benzene ring, it is named as 1,2-dibromobenzene.
3. When different substituted groups are attached to the aromatic compounds, the substituent of the base compound is assigned number one and then the direction of numbering is chosen such that the next substituent gets the lowest number. Substituents are named in alphabetical order. For example: when chloro and nitro groups are attached to the benzene ring, we first locate the chloro group then nitro groups.
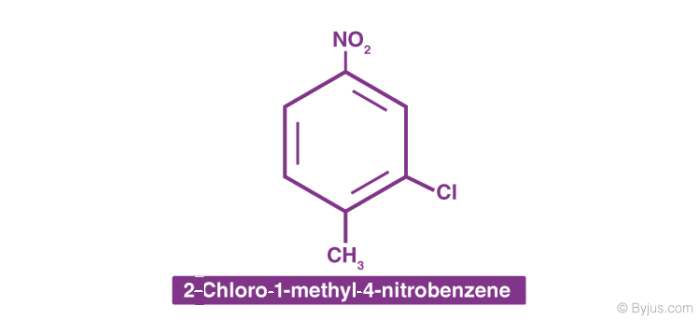
4. In the case of multiple substituted aromatic compounds, sometimes terms like ortho (o), meta (m) and para (p) are also used as prefixes to indicate the relative positions 1,2-; 1,3- and 1,4- respectively. For example, 1,2-Di-bromo-benzene can be named as o-di-bromo-benzene.
5. When an alkane with a functional group is attached to an aromatic compound, the aromatic compound is considered as a substituent, instead of a parent. For example: when a benzene ring is attached to an alkane with a functional group, it is considered as a substituent named phenyl, denoted by Ph-.
Frequently Asked Questions – FAQs
What is an aromatic organic compound?
Aromaticity is a property in organic chemistry of cyclic (chain-shaped), planar (flat) structures with a ring of resonance bonds that gives greater stability compared to other geometric or connective arrangements with the same collection of atoms.
What are heterocyclic aromatic compounds?
A heterocyclic compound is an organic compound where an atom other than carbon has substituted one or more of the carbon atoms in the molecule’s backbone. Nitrogen, oxygen, and sulphur are normal hetero atoms.
What is the general formula of aromatic compounds?
Aromatic hydrocarbons are compounds that contain benzene as part of their structure, also known as aromatic compounds. Benzene, with formula C6H6, is a cyclic hydrocarbon.
What is the difference between aliphatic and aromatic?
The carbon compounds are related in a straight chain way in aliphatic compounds. In aromatic compounds, the carbon compounds are associated with conjugated pi electrons in the manner of a ring structure.
Are all aromatic compounds cyclic?
Cyclic compounds may or may not be aromatic; benzene is an example of a cyclic aromatic compound, while cyclohexane is non-aromatic. Organic compounds that are not aromatic are known as aliphatic compounds, but only aromatic rings are especially stable.
What are the conditions for a compound to be aromatic?
What is Huckel’s rule of aromaticity?
Why are aromatic compounds more stable?
Why does conjugation increase the stability of the compound?
Are aromatics more reactive than alkenes?
For a detailed discussion on the nomenclature of alkanes, alkenes, and alkynes, download BYJU’S – the learning app.

Very good