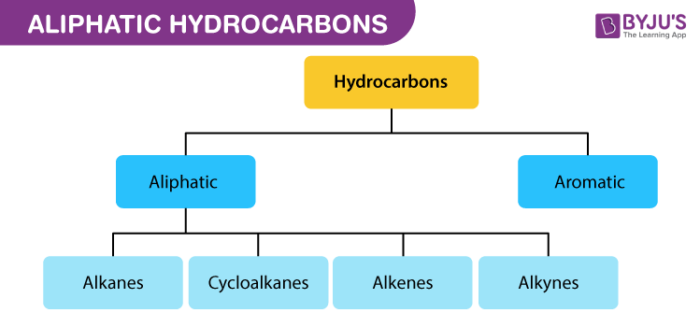
What is Aliphatic Hydrocarbon?
Halogenated aliphatic hydrocarbons are organic chemicals in which one or more hydrogen atoms have been replaced by a halogen (i.e., fluorinated, chlorinated, brominated or iodized).
Chlorinated Aliphatic Hydrocarbons constitute a diverse group of organic compounds characterized by an open-chain structure and a variable number of single, double, and triple bonds.
The term Aliphatic has been derived from the Greek word “Aleiphar” which translates to “fat”. It is used to describe hydrocarbons that are obtained by the chemical degradation of oils or fats.
Table of contents
- Aliphatic Hydrocarbon Definition
- Recommended Videos On Aliphatic Hydrocarbons
- Saturated And Unsaturated Aliphatic Hydrocarbons
- Properties Of Aliphatic Hydrocarbons
- Examples And List Of Aliphatic Hydrocarbons
- Extraction Of Aliphatic Hydrocarbons
- FAQs
Aliphatic Hydrocarbon Definition
An aliphatic compound or aliphatic hydrocarbon is an organic compound containing hydrogen and carbon atoms that are usually linked together in chains via single, double or triple bonds.
Sometimes the chains are also in branched trains or in the form of non-aromatic structures. Notably, apart from hydrogen some other elements like oxygen, nitrogen, chlorine, and sulphur may be bound to the carbon atoms in the chain.
Recommended Videos on Aliphatic Hydrocarbons

Saturated and Unsaturated Aliphatic Hydrocarbons
Aliphatic compounds may be saturated or unsaturated. Saturated hydrocarbon contains mainly alkanes which are open chain hydrocarbons containing a carbon-carbon single bond. Most of the time the bond exists in the form of a covalent bond. These compounds are inert in nature and do not readily react with acid, bases or other reagents.
Hydrocarbon molecules with at least one double bond are called unsaturated meaning that more hydrogen atoms can be added to these molecules. Such molecules are much more reactive than saturated Unsaturated ones. This is because the double bond is less than twice as strong as a single bond., making it easier to break one part of the double bond apart than it would be to break a single bond.
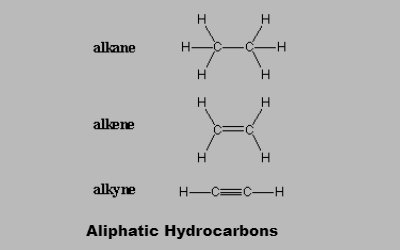
Hydrocarbon molecules which have no double bonds in them are called saturated. This simply means that there are as many hydrogen atoms as possible in the molecule, and no more can be added. Unsaturated hydrocarbons are alkenes and alkynes which have one carbon-carbon double bond and one carbon-carbon triple bond respectively. Unsaturated hydrocarbons are more reactive than saturated hydrocarbons, and usually fewer hydrogen atoms can be seen in bonds with carbon atoms.
Properties of Aliphatic Hydrocarbons
- Most aliphatic hydrocarbons are flammable. These compounds are used as fuels.
- Aliphatic compounds can be cyclic or acyclic meaning they can contain close chains or rings of carbon atoms in their molecule.
- Boiling point and melting point – The small difference between the electronegativities of carbon and hydrogen means that the bond between them is only very weakly polar. Chain branching causes a decrease in the area of contact. So if two alkanes have the same molecular weight the more highly branched one will have the lower boiling point. The melting points of aliphatic hydrocarbons also increase with size but in a less regular manner.
- Solubility and density – As the hydrocarbons are non-polar they tend to be insoluble in water and other polar solvents. They prefer to dissolve in non-polar solvents such as benzene and diethyl ether. Thus, hydrocarbons can be described as hydrophobic or lipophilic. The hydrocarbons are less dense than water meaning that they float on the surface of the water.
Examples and List of Aliphatic Hydrocarbons
| Number of Carbons | Aliphatic Hydrocarbons |
|---|---|
| 1 | methane |
| 2 | ethane, ethene, ethyne |
| 3 | propane, propene, propyne, cyclopropane |
| 4 | Methylpropane, butane, cyclobutene |
| 5 | cyclopentene, pentane, dimethylpropane, |
| 6 | hexane, cyclohexene, cyclohexane |
| 7 | cycloheptane, cycloheptene, heptane |
| 8 | octane, cyclooctane, cyclooctene |
Extraction of Aliphatic Hydrocarbons
Aliphatic compounds can be extracted by the process known as Pressurized Fluid Extraction or PFE where organic and aqueous extraction solvents are used. Water which is converted to hot steam can also be used to extract aliphatic hydrocarbons mostly from solid and semi-solid environmental samples.
Very little use has been made of aliphatic hydrocarbons as solvents in conventional flame spectrometry. Pentane, methyl cyclopentane and cyclohexane have been tested as solvents for the determination of nickel in an oxygen cyanogen flame. These solvents appeared to offer no striking advantages, although excellent sensitivity was obtained when pentane was used in AAS. Heptane, cyclohexane and cyclohexene have been investigated as possible solvents for the determination of beryllium by AFS. In both the oxygen acetylene and the nitrous oxide acetylene solutions. Aliphatic hydrocarbons are occasionally used as diluents for other solvents.
Frequently Asked Questions/ FAQS
What is aliphatic hydrocarbon used for?
Many of the aliphatic compounds are flammable, making the use of hydrocarbons as fuel, such as methane in Bunsen burners, as liquefied natural gas (LNG), and ethylene (acetylene) in welding.
Is benzene aliphatic or aromatic?
Both organic compounds are aromatic and aliphatic. This is a typical chemical structure of aromatic compounds with benzene rings that comprises six carbon atoms, cyclically bonded with alternating double bonds. Whereas benzene rings are not in the aliphatic.
Is alcohol an aliphatic compound?
Aliphatic compounds, like hexane, do not only have to be made of carbon and hydrogen. They can contain oxygen-like atoms too. Isopropanol is an example of one such compound containing a group of alcohol (OH) bound to an aliphatic carbon chain.
What are the 4 types of hydrocarbons?
The term Hydrocarbons means organic compounds that contain only hydrogen and carbon. Using this description would include four groups of hydrocarbons: alkanes, alkenes, alkynes and aromatic.
Is aliphatic hydrocarbon dangerous?
One noteworthy phenomenon is the tendency of chlorinated aliphatic hydrocarbons to “crack” when heated to create poison gases (hydrogen chloride and phosgene) that are directly and intensely hazardous to cardio respiratory function, such as carbon tetrachloride.
What products contain hydrocarbons?
Some of the household items containing hydrocarbons are cosmetics dependent on mineral oil, such as bath oil, creams, lotions, and maquillage removers. Other commodity categories include fuel additives, motor oil, and waterproofing agents.
What are the effects of hydrocarbons?
Such chemicals, as major components of oil, natural gas, and pesticides, lead to the greenhouse effect and climate change, deplete ozone, decrease plant photosynthesis, and raise human cancer and respiratory disorders.
What is the main source of hydrocarbons?
Hydrocarbons are organic compounds composed only of two elements (carbon and hydrogen), hence their name for the source. The crude oil is the primary source of hydrocarbons. It’s a lot of hydrocarbons. We can be classified into two main classes: aliphatic, and hydrocarbon aromatic.

Comments