What are Aromatic Hydrocarbons?
Aromatic Hydrocarbons are circularly structured organic compounds that contain sigma bonds along with delocalized pi electrons. They are also referred to as arenes or aryl hydrocarbons.
Table of Content
- Aromatic Hydrocarbons Explanation
- Recommended Videos
- Properties of Aromatic Hydrocarbons
- Reactions of Aromatic Hydrocarbons
- Uses of Aromatic Hydrocarbons
- Polycyclic Aromatic Hydrocarbons
- Frequently Asked Questions – FAQs
Aromatic Hydrocarbons Explanation
The aromatic hydrocarbons are “unsaturated hydrocarbons which have one or more planar six-carbon rings called benzene rings, to which hydrogen atoms are attached”. Many aromatic hydrocarbons contain a benzene ring (also referred to as an aromatic ring). The benzene ring is stabilized by resonance and the pi electrons are delocalized in the ring structure.
A few examples of aromatic hydrocarbons are provided below. It can be observed that all these compounds contain a benzene ring.
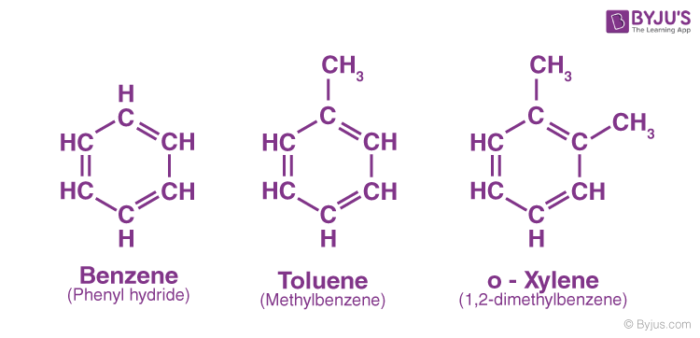
Check ⇒ Xylene
The aromatic hydrocarbons which do not contain a benzene ring are commonly referred to as heteroarenes. All of these heteroarenes obey Huckel’s rule (total number of pi electrons in a monocyclic ring = 4n + 2 where n is any positive integer or zero).
In these types of compounds, a minimum of one carbon is replaced by either nitrogen, oxygen, or sulphur. Common examples of heteroarenes include furan (contains oxygen) and pyridine (contains nitrogen).
Recommended Videos
Properties of Aromatic Hydrocarbons
“The first compound that was categorized as an aromatic hydrocarbon was benzene”. It is also the most complex aryl hydrocarbon. Each carbon atom belonging to the benzene ring has two carbon-carbon sigma bonds, one carbon-hydrogen sigma bond, and one double bond with a neighbouring carbon in which the pi electron is delocalized.
This delocalization of pi electrons in the benzene molecule is represented by a circle inside the hexagon. The bond order of all carbon-carbon bonds in this molecule is considered to be 1.5 and this equivalency can be explained with the help of the resonance structures of benzene.
Some general properties of aromatic hydrocarbons have been listed below.
- These compounds exhibit aromaticity (additional stability granted by resonance)
- The ratio of carbon atoms to hydrogen atoms is relatively high in these types of molecules.
- When burnt, the aromatic hydrocarbons display a strong and sooty flame which is yellow.
- These compounds generally undergo electrophilic substitutions and nucleophilic aromatic substitution reactions.
It can be noted that these compounds can be either monocyclic or polycyclic.
Reactions of Aromatic Hydrocarbons
Many organic chemical reactions involve the use of aromatic hydrocarbons as the primary reactant. Some such reactions are listed in this subsection along with a brief description of each of these reactions.
1. Aromatic Substitution Reactions
These reactions involve the replacement of one substituent on the ring of an aromatic hydrocarbon, commonly a hydrogen atom, by a different substituent group.
The common types of aromatic substitution reactions include:
- Nucleophilic aromatic substitution reactions
- Electrophilic aromatic substitution reactions
- Radical nucleophilic aromatic substitution reactions
An example of an aromatic substitution reaction is the electrophilic substitution observed in the nitration reaction of salicylic acid.
2. Coupling Reactions
In these types of reactions, the coupling of two fragments which have a radical nature is achieved with the help of a metal catalyst. When aromatic hydrocarbons undergo coupling reactions, the following type of bonds can be formed.
- Carbon-carbon bonds can be formed from the coupling reactions of arenes and products such as vinyl arenes, alkyl arenes, etc. are formed.
- The formation of carbon-oxygen bonds can occur in these reactions, forming aryloxy compounds.
- Carbon-nitrogen bonds can form in coupling reactions, giving products such as aniline.
An example of a coupling reaction involving aromatic hydrocarbons can be observed in the arylation of perfluorobenzenes, as illustrated below.
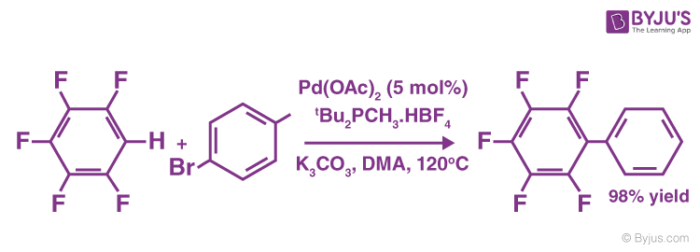
The catalyst used in this reaction is Palladium(II) acetate. It can also be noted that DMA is the abbreviation of Dimethylacetamide.
3. Hydrogenation Reactions
The hydrogenation reactions involving arenes generally lead to the formation of saturated rings. An example of such a reaction is the reduction of 1-naphthol into a mixture containing different isomers of decalin-ol.
Another example of such a reaction is the hydrogenation reaction of resorcinol with the help of spongy nickel (also referred to as Raney nickel) and aqueous NaOH. This reaction proceeds via the formation of an enolate, and the successive alkylation of this enolate (with methyl iodide) to yield 2-methyl-1,3-cyclohexanedione.
Uses of Aromatic Hydrocarbons
The use of aromatic hydrocarbons is common in both biological and synthetic processes. Some numerous uses of aromatic hydrocarbons are listed below.
- The green pigment found in plants, more commonly known as chlorophyll, consists of aromatic hydrocarbons and is very important in the process of food production in plants.
- The nucleic acids and amino acids in the human body also consist of these aromatic hydrocarbons.
- Methylbenzene which is an aromatic hydrocarbon is used as a solvent in model glues
- Naphthalene is an important item in the production of mothballs
- For the synthesis of drugs, dyes, and explosives, an aryl hydrocarbon known as Phenanthrene is used
- Trinitrotoluene or TNT is a very important aromatic hydrocarbon which is widely used for explosive purposes.
- Plastic industry and petrochemical industries make use of aromatic hydrocarbons extensively.
Polycyclic Aromatic Hydrocarbons
- These are the hydrocarbons which comprise aromatic rings in fused form. These are found in coal, tar, oil and some cooked foods such as smoked fish, burnt toast, etc.
- One common example of these polycyclic hydrocarbons is naphthalene. These compounds are said to be pollutants.
- Some examples of aromatic hydrocarbons are Methylbenzene, Naphthalene, Phenanthrene, Trinitrotoluene, and o-dihydroxybenzene.
Frequently Asked Questions – FAQs
Which is an aromatic hydrocarbon?
Aromatic hydrocarbons, often known as arenes, are aromatic organic molecules made up entirely of carbon and hydrogen. A “benzene ring,” named after the simple aromatic chemical benzene, or a phenyl group when part of a larger structure, is the configuration of six carbon atoms in aromatic compounds.
What is composed of aromatic hydrocarbons?
A single aromatic ring makes up monocyclic aromatic hydrocarbons (MAHs). The most volatile and water-soluble aromatic hydrocarbons include benzene, toluene, ethylbenzene, and xylenes (BTEX), which are well-known environmental contaminants.
What are aromatic substitution reactions?
Electrophilic aromatic substitution reactions occur when an electrophile substitutes an atom connected to an aromatic ring in an organic process. The substitution of a hydrogen atom from a benzene ring with an electrophile is common in these reactions.
How do aromatic compounds react?
Aromatic compounds, also known as arenes, go through substitution reactions in which the aromatic hydrogen is replaced by an electrophile, resulting in electrophilic substitution. Metal cross-coupling, such as the Suzuki reaction, allows two or more aromatic compounds to generate carbon-carbon bonds.
What is Sandmeyer’s reaction explain with an example?
The Sandmeyer reaction is a chemical reaction that uses copper salts as reagents or catalysts to manufacture aryl halides from aryl diazonium salts. A radical-nucleophilic aromatic substitution is an example.
To learn more about benzene and other aromatic hydrocarbons, register with BYJU’S and download our mobile application on your smartphone.

Comments