What are Binary Compounds?
Binary compounds are chemical compounds comprising of two distinct elements. An element is a substance that cannot be further divided into any simpler substances using chemical methods. Hence sodium fluoride, magnesium oxide, and calcium chloride are all chemical elements. So it implies that binary compounds comprise exactly two different chemical elements.
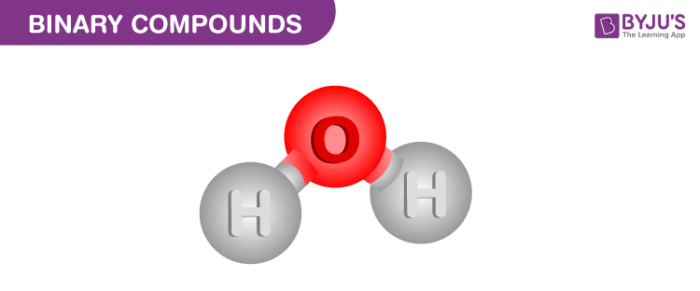
Table of Contents
Examples of Binary Compounds
There are binary compounds which consist of more than one of each element. For example water, it comprises of two hydrogen atoms and one oxygen atom (H2O). Water and calcium chloride are also examples of binary compounds that one can see in their daily life.
Binary Acids
It is a group of binary compounds of hydrogen which includes a hydrogen atom attached to another atom which is in the 7th group of the periodic table. These elements include astatine, fluorine, chlorine, iodine, and bromide. Other elements such as arsenic, sulfur, polonium, selenium and tellurium can also be considered.
The strength of binary acids depends on various factors namely electronegativity, bond strength and dissociation constant. Binary acids are much stronger than other types of acids.
The designated convention is: “Hydro-” + Nonmetal + “-ic” + “acid”
Example: Hydrochloric Acid (HCl).
Difference between Binary Acids and Binary Compounds
One of that factor that differentiates binary acids from binary compounds is when we consider its physical state. Acids are assigned the symbol ‘aq’ in aqueous molecules as acids are also present in aqueous solution. Symbol ‘l’ and ‘g’ stands for liquid or gas in a molecule than it is a binary compound.
How to name Binary compounds?
It is important to consider the following steps while naming the binary compound.
-
-
-
- The order followed to name in binary compounds is such that the cation is named first and then the anion.
- From the periodic table consider cation with a fixed oxidation state.
- While naming the anion, consider the element’s root name and add the suffix ‘-ide.’
-
-
Binary Ionic Compound
Binary ionic compounds are salts which consist of only 2 elements. Here both elements are ions (an anion which has a negative charge and a cation which has a positive charge). The binary compound list is mentioned in the table below.
Binary Ionic Compound List |
| Potassium fluoride |
| Copper (II) chloride |
| Lead (II) bromide |
| Lead (II) iodide |
| Copper (II) sulfide |
| Calcium sulfide |
| Lead (II) iodide |
| Iron (II) bromide |
| Sodium oxide |
| Lead (II) bromide |
| Sodium sulfide |
| Aluminum fluoride |
| Magnesium chloride |
| Aluminum chloride |
Stay tuned with BYJU’S to learn more interesting topics in Chemistry. Also, get various engaging and interactive video lessons to learn more effectively.
Frequently asked questions
Write the formula for binary compounds.
The formula for binary compounds is written as A+B→AB.
Water is a binary compound. True or false?
True.
Read more:

Comments