What is an Electron?
The electron is a low-mass, negatively charged particle. As such, it can easily be deflected by passing close to other electrons or the positive nucleus of an atom.
| Mass of Electron | m = mass of an electron in kg = 9.10938356 × 10-31 kilograms. |
| Charge of Electron | e = magnitude of the charge of an electron in coulombs = 1.602 x 10-19 coulombs. |
The history of atomic structure and quantum mechanics dates back to the times of Democritus, the man who first proposed that matter is composed of atoms. These theories could not gain much importance due to the lack of technology. The experiments conducted during the nineteenth and early twentieth centuries revealed that even an atom is not the ultimate particle. The continued efforts of the scientists led to the discovery of subatomic particles like electrons, protons, and neutrons.
In the nineteenth century, J.J Thomson proposed Thomson’s Atomic Model discovered the electron to mark the inception of the world of subatomic particles. Once the electron was discovered, he continued his experiments to calculate the charge and the mass of the electron. With the help of his experiments, he derived a formula for the calculation of the charge to mass ratio of the electron.
Charge to Mass Ratio of Electron
The charge to mass ratio of the electron is given by:
Where,
- m = mass of an electron in kg = 9.10938356 × 10-31 kilograms.
- e = magnitude of the charge of an electron in coulombs = 1.602 x 10-19 coulombs.
Experimental setup for the determination of charge to mass ratio of the electron
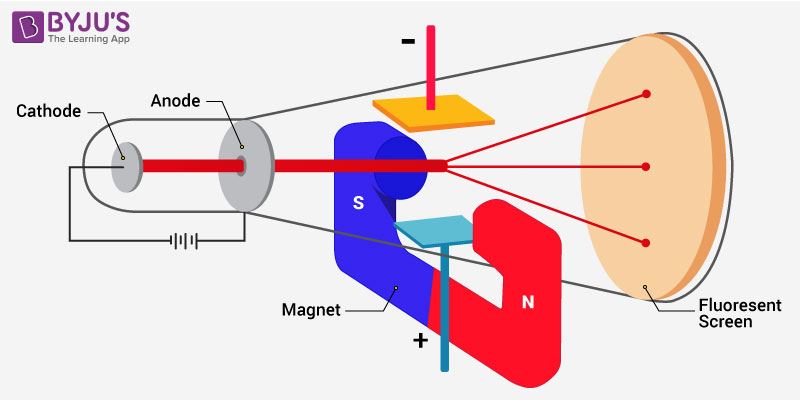
Diagramatic representation of charge to mass ratio of the electron
While carrying out the discharge tube experiment, Thomson observed that the particles of the cathode deviate from their path. He noticed the amount of deviation in the presence of an electrical or magnetic field depends on various related parameters. They are
- Particles with a greater magnitude of the charge experienced greater interaction with the electric or magnetic field. Thus, they exhibited greater deflection.
- Lighter particles experienced greater deflection. Thus, deflection is inversely proportional to the mass of the particle.
- Deflection of particle from their path is directly proportional to the strength of the electrical and the magnetic field present.
Frequently Asked Questions – FAQs
What is the mass of one proton?
The proton is a stable subatomic particle with a positive charge equal to that of an electron and a rest mass of 1.67262 1027 kg, or 1,836 times the mass of an electron.
Do Protons have mass?
Protons, neutrons, and electrons: The nucleus contains protons and neutrons, each of which has a mass of one amu. Protons, on the other hand, have a charge of +1, whereas neutrons are uncharged. Electrons have a charge of -1 and a mass of around 0 amu. They circle the nucleus and have a mass of roughly 0 amu.
What is an electron?
An electron is a subatomic particle with a negative charge. It can be either free (not bound to any atom) or tied to an atom’s nucleus. The energy levels of electrons in atoms are represented by spherical shells of varying radii. The unit electrical charge is defined as the charge on a single electron.
Where do protons get their mass?
These particles are made up of three quarks that are bonded together by gluons, the particles that convey the strong force, and move at dizzying speeds. The mass of protons and neutrons is determined by the energy of this interaction between quarks and gluons.
Who named Proton?
Ernest Rutherford discovered the proton in the early 1900s. During this time, his study led to the first splitting of the atom, when he found protons through a nuclear reaction. His finding was given the term “protons” from the Greek word “protos,” which means “first.”
To learn more about the charge to mass ratio of the electron, J J Thomsons Atomic Model and its limitations and more topics of chemistry, register with BYJU’S.

It was really helpfull.
Thanks a lot.
Thanks. I was searching for notes across the web.
its really good to understand this topic, its really made me easy to learn .thank you
really helpfull
thanx a lot