Table of Contents
What is Chlorine gas?
Chlorine is a green yellow gas with a very pungent odour that is twice as dense as air.
It is a chemical element that belongs to the halogen group with the symbol Cl. It was discovered in 1770’s and soon became useful as a commercial agent. It is found easily in its natural state. It should be handled carefully because it is poisonous at low concentrations. Chlorine is very harmful and has been used as a chemical weapon throughout history.
Other names – Molecular chlorine
| Cl2 | Chlorine gas |
| Density | 3.2 g/L |
| Molecular Weight/ Molar Mass | 70.90 u |
| Boiling Point | -34.04 °C |
| Melting Point | -101.5 °C |
| Chemical Formula | Cl2 |
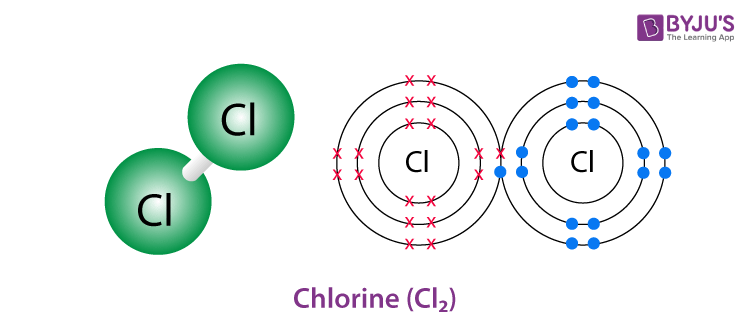
Chlorine gas Structure – Cl2
Physical Properties of Chlorine gas – Cl2
| Odour | Odour of bleach |
| Appearance | Yellow green gas |
| Covalently-Bonded Unit | 1 |
| Vapour Pressure | 85.3 psig |
| pH | 7.4 |
| Solubility | Slightly soluble in water |
Chemical Properties of Chlorine gas – Cl2
-
-
-
- Chlorine reacts with organic compounds and ammonia to form chloro-organics or chloramines. Chloramines are part of the group of chlorine compounds that have disinfectant properties and show up as part of the chlorine residue test.
- It acts as a reducing agent present in wastewater. These reactions are called chlorine demand. The reaction is as below.H2O + Cl2 → HCl + HClO
-
-
Uses of Chlorine gas – Cl2
-
-
-
- Chlorine gas was used by the Germans as a chemical weapon against the allied troops during the First World War.
- The most common use of chlorine in wastewater treatment is for disinfection.
- Used in odour control and in the control of filamentous organisms in the activated sludge process.
- Despite all these, it is used for preventing the spread of waterborne disease and the most common means of disinfection in use.
-
-
Frequently Asked Questions
How is chlorine gas produced?
The electrolysis of a sodium chloride solution (brine), known as the Chlor-alkali process, can be used to produce chlorine. Chlorine can also be formed by electrolysis of a potassium chloride solution, in which case the hydrogen and caustic potash are the co-products
How long does chlorine gas stay in the air?
The free residual chlorine dissipates rapidly as treated effluent is released through receiving waters.
How does chlorine gas affect the body?
Breathing elevated chlorine levels causes build-up of fluid in the lungs, a condition known as pulmonary edema. Development of pulmonary edema after exposure to chlorine can be delayed for several hours. Compressed liquid chlorine touch can cause skin and eyes to get frostbite.
Does chlorine gas dissolve in water?
At room temperature chlorine is a yellow-green gas. Chlorine is highly soluble in water and binds to hypochloric acid (HClO) and hydrochloric acid (HCl) with moisture.
How dangerous is chlorine gas?
Chlorine gas is an intermediate water-soluble pulmonary irritant that causes acute damage in the upper and lower respiratory tract. Currently, the greatest risk for severe toxicity from high concentration chlorine is occupational exposure.


Comments