What is Condensation?
Condensation is the process by which water vapour in the air is changed into liquid water. In other words, the water in the air, a gas known as water vapour.
Condensation is the transformation of water vapour into liquid water, and the finest illustration is those enormous, fluffy clouds floating over your head. When the water droplets in clouds mix, they become heavy enough to pour down.
Table of Contents
What is Condensation?
Condensation is the process through which the physical state of matter changes from the gaseous phase into the liquid phase. For example, condensation occurs when water vapour (gaseous form) in the air changes into liquid water when it comes in contact with a cooler surface. When the water in the air comes in contact with a cold surface, it condenses to form water droplets. The opposite of condensation is evaporation reaction.
Condensation Definition
Condensation can be alternately defined as follows:
- Condensation is when a gas turns to a liquid.
- Condensations have been defined to include those reactions in which two molecules are joined with loss of water.
- Condensation is defined as the removal of heat from a system in such a manner that vapour is converted into liquid.
In the summer, after working under the sun when we feel very thirsty. After reaching home, when we take a cold water bottle from the refrigerator, we can notice small droplets of water outside the bottle. Where did these water droplets come in the bottle? This happens due to a phenomenon called condensation.
Recommended Videos
Vapour Pressure

Condensation Reaction

Basic Process of Condensation
Condensation of water happens when water changes its phase from gaseous state to liquid or crystal shape. At high pressure and low temperature, any gas can condense. Technically, the process of condensation can happen at any temperature as long as the pressure of the liquid state of the gas is less than the pressure of the condensing gas. The molecules in the matter slow down during the process of condensation because the heat energy is taken away, which causes a change within the three states of matter, that is it changes the matter into the solid-state.
Condensation – Water Cycle
- Condensation is important to the water cycle as it is responsible for the formation of clouds.
- Water vapour present in the air is responsible for the formation of clouds which ultimately comes down in the form of rain.
- This phase change of water between solid, liquid and gas is because of the movement of water molecules.
- In vapour form, water molecules are arranged randomly as compared to the liquid state.
- As condensation happens, water molecules become more organized and as a result, heat is released into the atmosphere leading to a change of phase from the vapour state to the liquid state.
- This generally occurs in the atmosphere when warm air rises up and cools down.
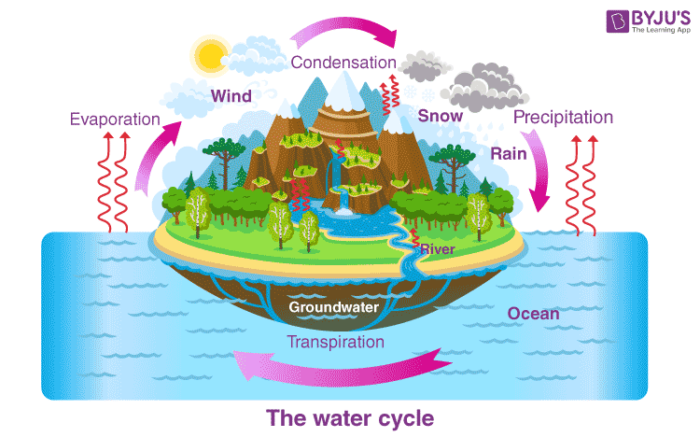
For condensation to take place, it is very important that the atmosphere is fully saturated (to reach maximum vapour pressure). Usually, condensation takes place around dust particles or smoke or microscopic bacteria. It plays a very significant role in the water cycle and thus helps in maintaining the water balance in the environment. It is also used in various industrial processes by the scientists and engineers for separating mixtures and manufacturing pure substances.
Frequently Asked Questions – FAQs
What causes condensation?
What is a good example of condensation?
Where is condensation used?
How is condensate formed?
Is condensation physical or chemical?
Stay tuned with BYJU’S to learn more interesting topics in Chemistry. Also, get various engaging and interactive video lessons to learn more effectively.
Also, Read:
| Aldol Condensation | Difference Between Evaporation and Condensation |

Comments