| Abstract |
| Understand the water cycle definition |
| Discover the water cycle steps |
| Explore the implications of the water cycle on the environment |

What is the Water Cycle?Water Cycle DiagramStages of Water CycleImplications of Water CycleFrequently Asked Questions
What is the Water Cycle?
The water cycle, also known as the hydrologic cycle or the hydrological cycle, describes the continuous movement of water on, above and below the surface of the Earth.

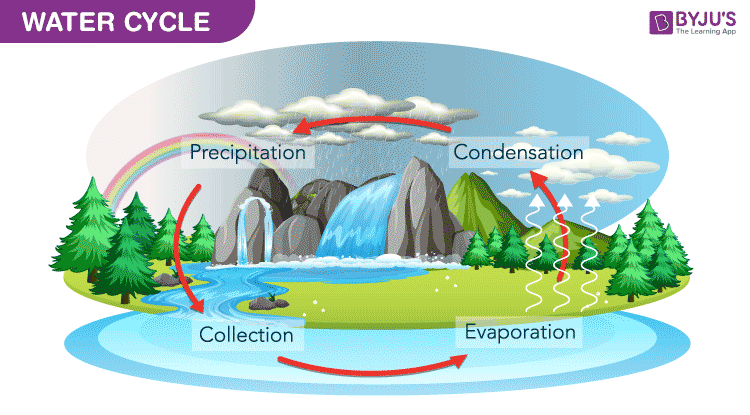
Water Cycle Diagram
During this process, water changes its state from one phase to another, but the total number of water particles remains the same. In other words, if it were possible to collect and boil 100 gms of water, it will still retain a mass of 100 gms as steam. Likewise, if 100 gms of steam is collected and condensed, the resultant water would still weight 100 gms.
Water changes its state through a variety of processes from evaporation, melting and freezing, to sublimation, condensation, and deposition. All these changes require the application of energy.
Stages of Water Cycle
There are many processes involved in the movement of water apart from the major steps given in the above water cycle diagram. Listed below are different stages of the water cycle.
1. Evaporation
The sun is the ultimate source of energy, and it powers most of the evaporation that occurs on earth. Evaporation generally happens when water molecules at the surface of water bodies become excited and rise into the air. These molecules with the highest kinetic energy accumulate into water vapour clouds. Evaporation usually takes place below the boiling point of water. Another process called evapotranspiration occurs when evaporation occurs through the leaves of plants. This process contributes to a large percentage of water in the atmosphere.
2. Sublimation
Sublimation occurs when snow or ice changes directly into water vapour without becoming water. It usually occurs as a result of dry winds and low humidity. Sublimation can be observed on mountain peaks, where the air pressure is quite low. The low air pressure helps to sublimate the snow into water vapour as less energy is utilised in the process. Another example of sublimation is the phase where fog bellows from dry ice. On earth, the primary source of sublimation is from the ice sheets covering the poles of the earth.
3. Condensation
The water vapour that accumulated in the atmosphere eventually cools down due to the low temperatures found at high altitudes. These vapours become tiny droplets of water and ice, eventually coming together to form clouds.
4. Precipitation
Above 0 degrees centigrade, the vapours will condense into water droplets. However, it cannot condense without dust or other impurities. Hence, water vapours attach itself on to the particle’s surface. When enough droplets merge, it falls out of the clouds and on to the ground below. This process is called precipitation (or rainfall). In particularly cold weather or extremely low air pressure, the water droplets freeze and fall as snow or hail.
5. Infiltration
Rainwater gets absorbed into the ground through the process of infiltration. The level of absorption varies based on the material the water has seeped into. For instance, rocks will retain comparatively less water than soil. Groundwater can either follows streams or rivers. But sometimes, it might just sink deeper, forming aquifers.
6. Runoff
If the water from rainfall does not form aquifers, it follows gravity, often flowing down the sides of mountains and hills; eventually forming rivers. This process is called runoff. In colder regions, icecaps form when the amount of snowfall is faster than the rate of evaporation or sublimation. The biggest icecaps on earth are found at the poles.
All the steps mentioned above occur cyclically with neither a fixed beginning nor an end.
Also Read: Back to the Oceans
Implications of Water Cycle
- The water cycle has a tremendous impact on the climate. For instance, the greenhouse effect will cause a rise in temperature. Without the evaporative cooling effect of the water cycle, the temperature on earth would rise drastically.
- The water cycle is also an integral part of other biogeochemical cycles.
- Water cycle affects all life processes on earth.
- The water cycle is also known the clean the air. For instance, during the process of precipitation, water vapours have to attach themselves on to particles of dust. In polluted cities, the raindrops, apart from picking up dust, also pick up water-soluble gas and pollutants as they fall from the clouds. Raindrops are also known to pick up biological agents such as bacteria and industrial soot particles and smoke.
Read more about the water cycle with diagram by registering @ BYJU’S Biology
Frequently Asked Questions
What are the major 4 steps in the water cycle?
The major 4 steps are evaporation of water, then condensation, precipitation and collection. The sun evaporates water sources and contributes to the formation of water vapor. These water vapour accumulate in the atmosphere as clouds. The vapours condense into water droplets and when enough droplets merge, it falls out of the clouds as rain.
What is the difference between evaporation and condensation?
Evaporation is a process by which water changes into water vapour. Condensation is an opposite process by which water vapour is converted into tiny droplets of water.
Why is water cycle important?
Water cycle has a huge impact on determining the global climate. It is also an integral part of other biogeochemical cycles. It affects all life processes on Earth either directly or indirectly.


Hello fellow human creatures of Earth. This online service has provided me with much needed information.
This is helpful
Helpful to me
Super useful
It is useful for me
This really Helped me!!
Thank you for the valuable information.
Educational, thanks!
It’s really helpful for my child Thanks from heart and soul
IT is VERY USEFUL
It’s very useful thank you