We all have seen our mothers separating stones and husk from rice and pulse grains in the kitchen. The visible impurities like stones and husk are easily removed by handpicking. But what do they do for smaller impurities like mud and dust? We notice that water is poured into the vessel and the upper layer of dirty water is discarded. The process of separation of solid impurities from the liquid solution is termed as decantation.
Table of Content
Decantation Definition
Decantation is the process of separation of liquid from solid and other immiscible (non-mixing) liquids, by removing the liquid layer at the top from the layer of solid or liquid below. The process can be carried out by tilting the mixture after pouring out the top layer. This process can also be used to separate two liquids that do not mix with each other for e.g., oil and water. When we leave the mixture of oil and water, two separate layers are formed, with water at the bottom and oil, being lighter, at the top. We can remove the oil layer from the top by pouring it into another vessel, which leaves us with the water layer at the bottom.

Usually this process is not very efficient in this separation, as thin layer of the remaining oil cannot be easily procured from the mixture. In order to make the procurement easier and the separation efficient, we use a separating funnel, as shown in the next figure.

Here, the bottom layer is collected first and the layer above it is made to remain in the vessel with the help of a stop cock, as shown which can be procured later.
Hence, separation of mixtures by this process is highly efficient if one of the components of mixture settles easily. But what can we do for the mixture where the particles do not settle. For e.g., for a mud water mixture, even after the process of decantation is complete, the water sample still looks slightly muddy. For such mixtures we use the process of loading.
Loading Definition
Loading is the process by which the mixture of liquids and liquids containing tiny impurities is separated by adding a chemical that sticks to the impurities and makes them heavier.
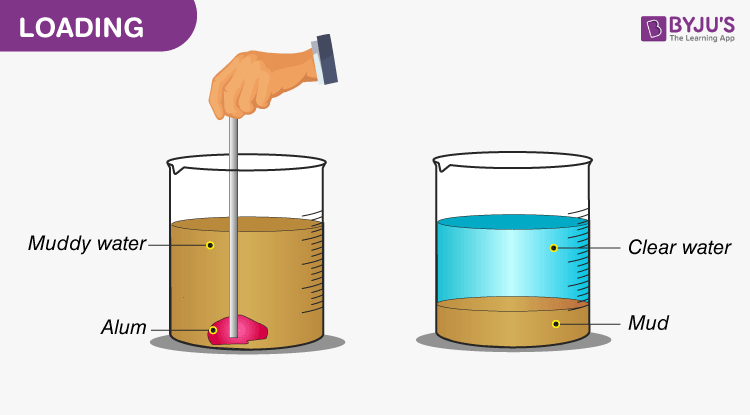
For e.g., if we take a slightly muddy sample and add some alum to it, after some time we observe that a layer of mud settles at the bottom of the vessel and water becomes clear. Here, the alum got adhered to the small bud particles and made it heavier, thus causing the mud to settle at the bottom. This process is used during the filtration of water in water filtration tanks.

To learn more about this topic, download BYJU’S – The Learning App from Google Play Store and watch interactive videos. Also, take free tests to practice for exams.
Frequently Asked Questions – FAQs
What is decantation?
Decantation is the process of separation of liquid from solid and other immiscible (non-mixing) liquids, by removing the liquid layer at the top from the layer of solid or liquid below.
What is loading?
Loading is the process by which the mixture of liquids and liquids containing tiny impurities is separated by adding a chemical that sticks to the impurities and makes them heavier.
How does loading help in decantation?
Loading helps in decantation. For example, when Alum is added to dirty water the dirt particles get loaded to form big aggregates which settle down quickly.
What is the limitation of decantation?
Decantation can not separate immiscible solutions. For example, we can not separate saltwater by decantation.
What is filtration?
Filtration is the separatory method in which solid particles in a liquid are removed by the use of filter paper. The solid particles retain on the filter paper while the fluid passes through the filter paper.

Thanks alot
This is so helpful
Very helpful. Thanks 😊
thanks it was very helpful
very helpful video
very good defination useful
useful
This video is so useful
Nice video
Literally, very useful definitions and self explanatory videos. Thank you very much!!