Electronic Configuration of Elements
“An atom of an element has different shells, sub-shells and orbitals and the distribution of electrons in these shells is called the electronic configuration.”
Representation of Electronic Configuration
Electronic configuration of any orbital can also be represented by simple notation nlx,
where,
- n = number of main or principle shell or the principal quantum number
- l = symbol of sub-shell or orbital
- x = no. of electrons present in that orbital
For example, 3p2 means that the p sub-shell in the 3rd main shell contains 2 electrons.
In order to fetch the full electronic configuration of an atom, these notations are written one after the other in the increasing order of the orbital’s energy. The filling of electrons in orbitals always starts from the orbital having the lowest energy with the electrons occupying the lower energy orbitals first.
Electronic configuration for few elements
Electronic configuration of few elements is given below:
- Helium = 1s2
- Beryllium = 1s22s1
- Carbon = 1s22s22px12py1
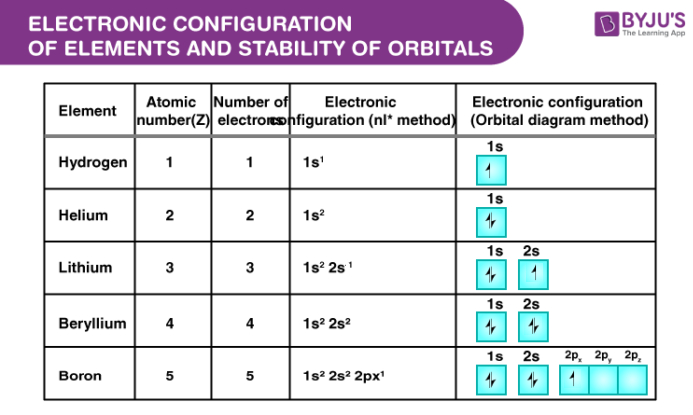
How to make electronic configuration simple?
In order to make the electronic configuration of the elements simple and convenient, it is written such that the electronic configuration of the noble gas core precedes the valence shell electrons. It is represented by the symbol of noble gases using square brackets followed by the valence shell configuration. For magnesium, the electronic configuration is written as [Ne]3s2 and calcium is represented as [Ar]4s2. There are a certain set of rules and principles that govern the filling of electrons in an orbital.
Recommended Videos

Stability of Completely Filled and Half-filled Orbitals
Almost all the elements follow the same trend for writing electronic configuration. Sometimes when two sub-shells differ in the energies, an electron from the lower energy moves to higher energy.
This is because of two reasons:
- Symmetrical distribution: As everyone knows that symmetry leads to stability. The orbitals in which the sub-shell is exactly half-filled or completely filled are more stable because of the symmetrical distribution of electrons.
- Exchange energy: The electrons which are there in degenerate orbitals have a parallel spin and tend to exchange their position. Exchange energy is nothing but the energy released during this process. When the orbitals are half-filled or completely filled then the number of exchanges is maximum. Therefore, its stability is maximum.
The knowledge about the electronic configuration of elements has paved the way for various further developments in the field of quantum mechanics. Learn more about the electronic configuration of elements with the expert faculty at BYJU’S.

Comments