Atomic spectra is the study of atoms (and atomic ions) through their interaction with electromagnetic radiation. We all know about the refraction of light. When light travels from one medium to another, it either bends towards the normal or away from the normal. The phenomenon of refraction is mainly attributed to the difference in the speed of light in various mediums. The speed of light depends upon the nature of the medium through which it passes.
Table of Contents
Let us understand the phenomenon of dispersion of white light through a prism. Also, learn about the emission spectrum and absorption spectrum.
Emission Spectrum
- Whenever electromagnetic radiation interacts with atoms and molecules of matter, the electrons in these atoms may absorb energy and jump to a higher energy state, losing their stability.
- In order to regain their stability, they need to move from the higher energy state to the previous lower energy state.
- To accomplish this job, these atoms and molecules emit radiation in various regions of the electromagnetic spectrum.
- This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as an emission spectrum.
Absorption Spectrum
- We observe that when a ray of white light falls on a prism it experiences refraction twice.
- Once when it travels from the rarer medium (air) to a denser medium (glass) and again from the denser medium (glass) to a rarer medium (air).
- Finally, we observe a band of colours, called a spectrum, formed out of a ray of white light. If we observe this spectrum more closely, the colour having a smaller wavelength deviates the most and vice versa.
- Thus, a spectrum of colours ranging from red to violet is observed where red having the longest wavelength suffers the least deviation.
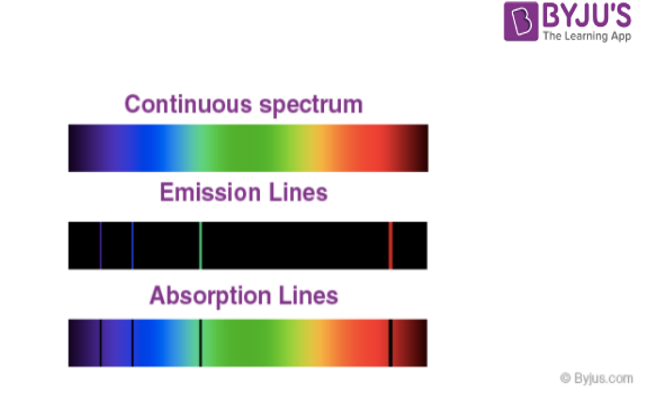
- This kind of spectrum is called a continuous spectrum as violet merges into blue, blue into green and so on.
However, the emission spectrum of atoms in the gas phase do not exhibit a continuous spread of wavelength from one colour to others. Rather, the emitted light consists of a specific wavelength having dark spaces existing between them. Such kind of spectra is known as atomic spectra or line spectra.
Recommended Videos
Spectrum Definition

Photoelectric Effect

Emission and Absorption Spectrum
Emission Spectrum & Absorption Spectrum
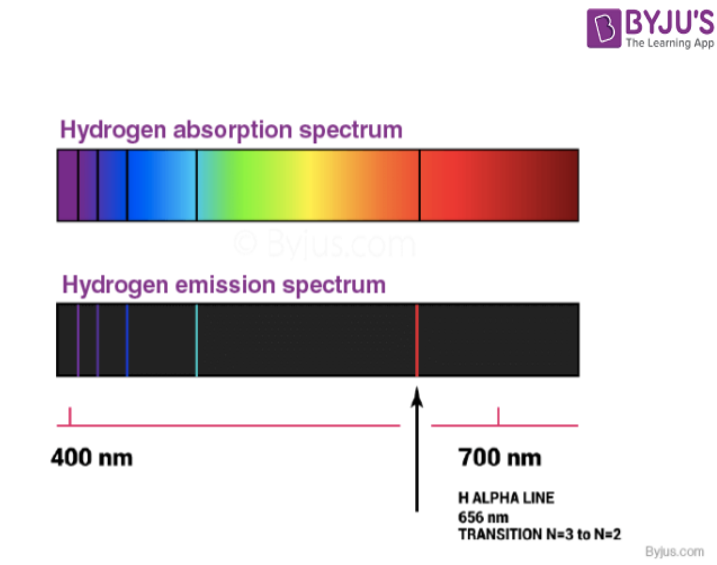
- An absorption spectrum is like a photographic negative of an emission spectrum.
- For observing the absorption spectrum, electromagnetic radiations are bombarded on a sample that absorbs radiation of certain wavelengths.
- The wavelength of radiation absorbed by the matter contributes to the missing wavelength which leaves dark spaces in the bright continuous spectrum.
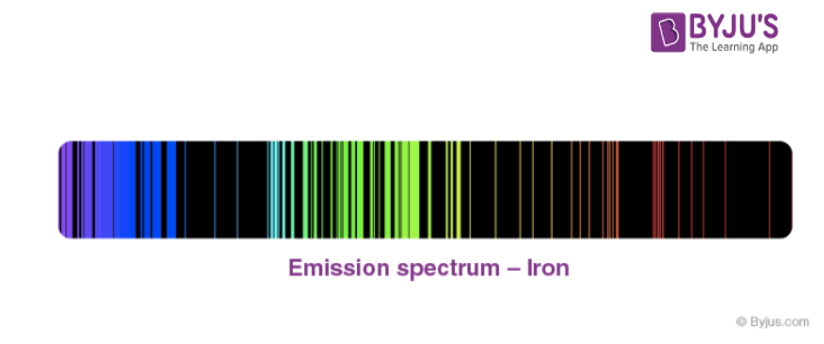
- Each element has its unique line emission spectrum. The study of the emission spectrum or absorption spectrum is better known as spectroscopy.
To learn more about the emission spectrum download BYJU’S – The Learning App.


Comments