The formaldehyde formula is given here along with its structure. Formaldehyde is also known as methanal or formalin, is an organic compound that occurs naturally. It is the simplest aldehyde and is colourless. Click on aldehydes and ketones to learn more about aldehydes, their uses, and occurrences.
Formaldehyde Formula
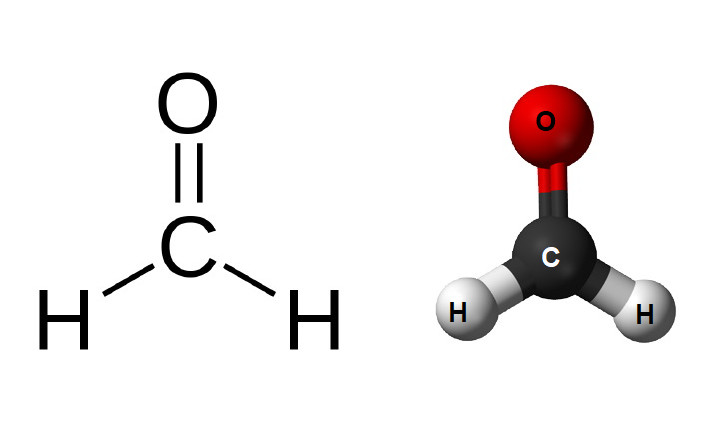
Formaldehyde, being the simplest aldehyde, has a very simple structure. Its molecule consists of a carbon atom from which two hydrogen atom is attached by a single bond and an oxygen atom bonded to the carbon atom by a double bond. The detailed chemical and structural formula for formaldehyde (methanal) are given in the following points.
Chemical Formula for Formaldehyde
Formaldehyde has a molar mass of 30.026 g/mol. The chemical formula of formaldehyde is given as-
| Formaldehyde Chemical Formula: CH2O |
Structural Formula for Formaldehyde
The carbon atom in methanal has sp2 hybridization and has planar-trigonal geometry. The structural formula of formaldehyde is-
There are several uses of formaldehyde and the most notable ones are that it is used in industries, in pharmaceuticals, constructions, food industry, etc. It is mainly prepared by the oxidation of methanol and is hard to find in nature as it is easily decomposed by sunlight.
To know about the chemical formulas of different other compounds, keep visiting BYJU’S.

Comments