What is Fractional Distillation?
Fractional distillation is a type of distillation which involves the separation of miscible liquids. The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.
Table of Contents
- Principle of Fractional Distillation
- Fractional Distillation Procedure
- Fractional Distillation of Crude Oil
- Recommended Videos
- Applications of Fractional Distillation
- Frequently Asked Questions – FAQs
Principle of Fractional Distillation
- Normally, the vapour composition of any liquid mixture does not remain equal to the liquid composition. When the mixture is heated, the liquid with the lower boiling point boils and converts to vapours.
- The more volatile component remains in a vapour state longer than the liquid component. Repeated distillations and condensations are used in the process, and the mixture is usually separated into component parts.
- The more volatile components increase in a vapour state after heating, and when this vapour is liquefied, the more volatile components increase in a liquid state.
- Distillation refers to the process of vaporisation followed by condensation (liquefaction). When this distillation process is repeated, a more volatile component will remain in a pure state in the liquid state. By using the fractional distillation method, components of the liquid-liquid mixture can be separated as a pure substance.
The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. So when the mixture is heated, the substance with lower boiling point starts to boil first and convert into vapours.
Fractional Distillation Procedure
Few fractional distillation apparatuses are required for the process. It includes distilling flask, condenser, receiver, fractionating column, thermometer and heat source.
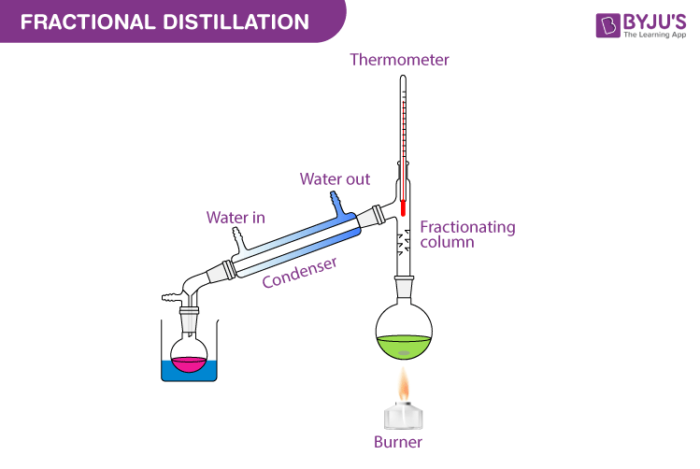
After setting up the apparatus, a mixture of two miscible liquids A and B is taken where A has more volatility than substance B. The solution is added into the distilling flask while the fractionating column is connected at the tip of the flask. Heat is applied which increases the temperature slowly. The mixture then starts to boil and vapours start rising in the flask. The vapours are from the volatile component A. The vapours then start moving through the fractionating column into the condenser where it is cooled down to form a liquid which is collected in the receiver.
Throughout the process, vaporization and condensation take place repeatedly until the two mixtures are separated completely.
Industrial Distillation
Fractional distillation is one of the popular separation techniques used in several industries. While the principle behind the process remains the same, the distillation is carried out on a larger scale. Usually, huge vertical cylindrical columns are known as “distillation columns” or “distillation or fractionation towers” are used. These industrial towers use reflux which ensures complete separation of the mixtures.

Fractional Distillation of Crude Oil
A common example of fractional distillation in industries is the separation of various components of crude oil. Crude oil normally contains substances such as paraffin wax, gasoline, diesel, naphtha, lubricating oil and kerosene. The distillation process helps in separating these components effectively.
Crude oil is added to the chamber and is heated with high-pressure steam. The mixture starts boiling and vapour is formed. At this point, various substances enter into the vapour phase. The vapour rises up in the fractional distillation column which consists of several plates. The plates have holes that allow the vapour to pass through them. The temperature is usually kept low at the top of the fractionating column. Here, components with the highest boiling point will condense in the lower part of the column while substances with a low boiling point will condense at the top. The condensed vapours or liquid fractions are then removed from the sides of the column. The collected liquid fractions can further be passed through condensers to cool them even more.
Recommended Videos
Types of DIstillation

Applications of Fractional Distillation
- Fractional distillation is used for the purification of water as well as for separating ethanol and water.
- Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds.
- Fractional distillation is also used for the separation of (liquefied) air. Components like liquid nitrogen and oxygen as well as concentrated argon are obtained.
- Distillation is used in the production of high-purity silicon from chlorosilanes. The silicon is widely used in semiconductors.
Frequently Asked Questions on Fractional Distillation
Which liquids are distilled using the fractional distillation method?
Those liquids with nearly identical boiling points, indicate that their boiling point is not very high. In this method, such liquids are used.
Identify the two critical conditions for fractional distillation.
The conditions for fractional distillation are-
- The liquids must be miscible with each other.
- The difference between the boiling points of the two liquids must be less than 25 degrees Celsius.
What are the steps involved in Fractional distillation?
The steps of the process are:
- Evaporation
- Condensation
- Collection
What is the difference between simple and fractional distillation?
Simple distillation is used to separate substances in mixtures with widely disparate boiling points, whereas fractional distillation is used for mixtures containing chemicals with similar boiling points.
What is the principle of fractional distillation?
Different liquids boil and evaporate at different temperatures, which is the basic principle of this type of distillation. As a result, when the mixture is heated, the substance with the lower boiling point begins to boil first, converting to vapours.
To learn more about fractional distillation, download BYJU’S – The Learning App.

Very good and nice app
Good answer .
If I have a doubt in my work i just know where to look.