What is Glucose?
Glucose is a simple sugar with six carbon atoms and one aldehyde group. This monosaccharide has a chemical formula C6H12O6.
It is also known as dextrose. It is referred to as aldohexose as it contains 6 carbon atoms and an aldehyde group. It exists in two forms, open-chain or ring structure. It is synthesized in the liver and kidneys of animals. In plants, it is found in fruits and in different parts of plants. D- glucose is the naturally occurring form of glucose. It can occur either in solid or liquid form. It is water-soluble and is also soluble in acetic acid. It is odourless and sweet to taste. In the year 1747, Andreas Marggraf, a German chemist, isolated glucose from raisins. In the year 1838, Jean Baptiste Dumas coined the word glucose.
Table of Content
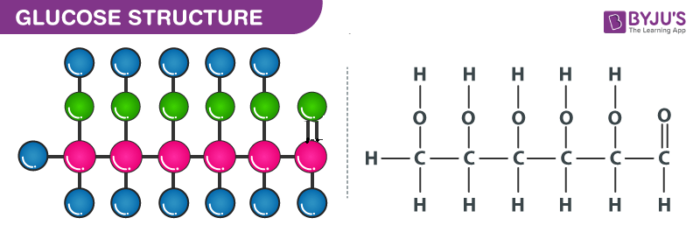
Structure of Glucose – C6H12O6
Properties of Glucose – C6H12O6
| C6H12O6 | Glucose |
| Molecular Weight/ Molar Mass | 180.16 g/mol |
| Density | 1.54 g/cm³ |
| Melting Point | 146 °C |
| Simple sugar | Monosaccharide |
Glucose can be called as aldohexose as well as dextrose. It is a monomer of many larger compounds such as carbohydrates, starch, and cellulose. On earth, this is the most abundant organic compound. On the basis of the following evidence, it was assigned the structure illustrated above:
- It has a molecular formula of C6H12O6
- When HI is heated for a long time, n-hexane is formed which indicates that all the six carbon atoms are linked in a straight chain.
- The oxime is formed when glucose reacts with hydroxylamine and cyanohydrins on the addition of hydrogen cyanide to it. This reaction can confirm the presence of the carbonyl group in glucose.
- On the reaction of glucose with a mild oxidizing agent like bromine water, the glucose gets oxidized to a carboxylic acid that contains six carbon atoms. This indicates that the carbonyl group is present as an aldehyde group.
- The presence of -OH group is confirmed after the acetylation of glucose with acetic acid, which gives glucose pentaacetate.
- Glucose as well as gluconic acid both yields dicarboxylic acid and saccharic acid on oxidation with nitric acid. The presence of primary alcohol is indicated by this.
Preparation Of Glucose (C6H12O6)
Sucrose (cane sugar) and starch are the two major sources of Glucose.
Preparation from sucrose or cane sugar:
Sucrose is a disaccharide with the formula C12H22O11. On boiling an aqueous solution of sucrose with dilute HCl or dilute H2SO4, Glucose and Fructose are formed in equimolar proportions.
C12H22O11 + H2O → C6H12O6 + C6H12O6
Sucrose Glucose Fructose
Preparation from starch:
It is a polysaccharide that when boiled with dilute H2SO4 at 393 K under 2 to 3 atmosphere pressure, gives glucose.
( C6H12O5)n + n H2O → nC6H12O6
Starch Glucose
Uses Of Glucose (C6H12O6)
- It is used in the treatment of hypoglycemia (low blood sugar)
- It is given to patients who are very sick and cannot eat as it provides carbohydrate calories
- It is used in the treatment of increased potassium levels in the blood (hyperkalemia)
- It is used as a precursor for the synthesis of substances.
Frequently Asked Questions- FAQs
How do you represent glucose?
The chemical formula of Glucose is C6H12O6. Glucose is a monosaccharide containing an aldehyde group (-CHO). It is made of 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms. Glucose is an aldohexose.
Is glucose a reducing sugar?
Glucose is a reducing sugar because it belongs to the category of an aldose meaning its open-chain form contains an aldehyde group. Generally, an aldehyde is quite easily oxidized to carboxylic acids.
What are the 5 reducing sugars?
The 5 reducing sugars are ribose, glucose, galactose, glyceraldehyde, xylose.
What are the elements of glucose?
Glucose consists of three elements such as carbon, hydrogen and oxygen. 6 carbon atoms bonded together as a chain with additional atoms of oxygen and hydrogen.
What type of sugar is glucose?
Glucose is a monosaccharides sugar. It occurs in the free state in the ripe grapes ( grape sugar) in honey and also many sweet fruits. Glucose is an essential constituent of human blood which normally contains 65 mg to 110 mg of glucose per 100 mL. It is named as blood sugar.
Also, Read:
| Difference Between Glucose and Fructose | Oxalic acid |
| Carbohydrates | Disaccharides |
Learn more about the isomerism, L-glucose, D- glucose and the structure of C6H12O6 from the expert faculties at BYJU’S.

Excellent explaination of the topic… nice…
Excellent