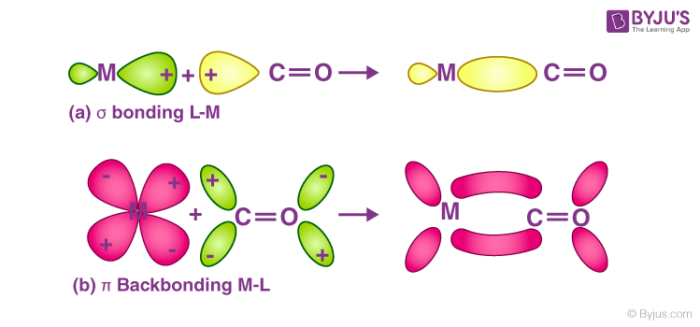
Till now, we have come across many complex compounds that contain carbonyl ligands only. These compounds are better termed as homoleptic carbonyls or metal carbonyls. Most of the transition metals are known to form homoleptic carbonyls or metal carbonyls which have simple, well-defined structures. For example tetrahedral, octahedral etc. In a metal carbonyl, the metal-carbon bond possesses both the σ and π character. The bond between the carbonyl molecule and the metal is further strengthened by the synergic effect produced by the metal-ligand bond. The two types of bonding that exist in metal carbonyls are explained below:
Structure of Metal Carbonyls:
- Due to the donation of electrons by the carbonyl molecules to the vacant orbitals of the metal, a metal-carbon σ bond is formed.
- Due to the donation of a pair of electrons from a filled d orbital metal into the vacant anti-bonding π* orbital of carbonyl ligand, a metal-carbon π bond is formed.
Stability of Coordination Compounds:
Coordination compounds are found to dissociate in various solutions. The stability of a coordination compound in a solution mainly depends on the degree of association between the two species involved in the state of equilibrium. Quantitatively the stability of any complex is given by the magnitude of the equilibrium constant for the formation of the compound. A general example is given below:
A + 4B→ AB4
Thus, the amount of AB4 molecule in the solution depends upon the value of the equilibrium constant, k. This is also known as the stability constant. On the other hand, the instability constant or the dissociation constant of complexes is given by the reciprocal of the equilibrium constant of the formation reaction.
To learn more about metal carbonyls, complex compounds, coordination chemistry and inorganic chemistry with the vibrant video lessons, download BYJU’S – The Learning App.


Comments