Table of Contents
AimTheoryMaterials RequiredApparatus SetupProcedureObservationsCalculationsResults and DiscussionPrecautionsViva Questions
The titration of potassium permanganate (KMnO4) against Mohr salt is an example of redox titration. In close proximity to the endpoint, the action of the indicator is analogous to the other types of visual colour titrations in oxidation-reduction (redox) titrations.
Aim:
To determine the strength of a given potassium permanganate solution against a standard ferrous ammonium sulfate (Mohr’s salt) solution.

Theory:
Potassium permanganate is a strong oxidant in the presence of sulfuric acid. Mohr salt is a double salt forming a single crystalline structure having the formula FeSO4.(NH4)2SO4.6H2O. The chemical name for Mohr’s salt is ferrous ammonium sulfate.
In this titration Mohr salt acts as a reducing agent and potassium permanganate acts as an oxidising agent. So, the reaction between Mohr’s salt and potassium permanganate is a redox reaction. In this redox reaction, ferrous ion from Mohr’s salt gets oxidised and pink coloured of manganese present in potassium permanganate, which is in the +7 oxidation state gets reduced to colourless Mn2+ state.
The chemical reaction and the molecular chemical equation is given below.
Reduction half reaction –
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
Oxidation half reaction –
[2FeSO4(NH4)2SO4.6H2O + H2SO4 + [O] → Fe2(SO4)3 + 2(NH4)2SO4 + 13H2O] x 5
Overall reaction –
2KMnO4 + 10FeSO4(NH4)2SO4.6H2O+ 8H2SO4 → K2SO4+ 2MnSO4+ 5Fe2(SO4)3+ 10(NH4)2SO4+ 68H2O
The ionic equation involved in the process is given below.
Oxidation half reaction – [Fe2+ → Fe3+ + e–] x 5
Reduction half reaction – MnO4– + 8H+ + 5e– → Mn2+ + 4H2O
Overall ionic equation – MnO4– + 8H+ + 5Fe2+ → Mn2+ + 5Fe3+ + 4H2O
This titration is based upon oxidation-reduction titrations. When ferrous ammonium sulfate solution is titrated against potassium permanganate in the presence of acidic medium by sulfuric acid. Acidic medium is necessary in order to prevent precipitation of manganese oxide. Here KMnO4 acts as a self indicator and this titration is called permanganate titration.
| Also Read: Mohr Salt Titration with KMnO4 Viva Questions |
Materials Required:
- Mohr’s salt (ferrous ammonium sulfate)
- Potassium permanganate solution
- Dilute sulfuric acid
- Chemical balance
- Burette
- Burette stand
- Pipette
- Conical flask
- Funnel
- Measuring flask
- Weighing bottle
- White tile
- Burnet
- Wire gauze
Apparatus Setup:
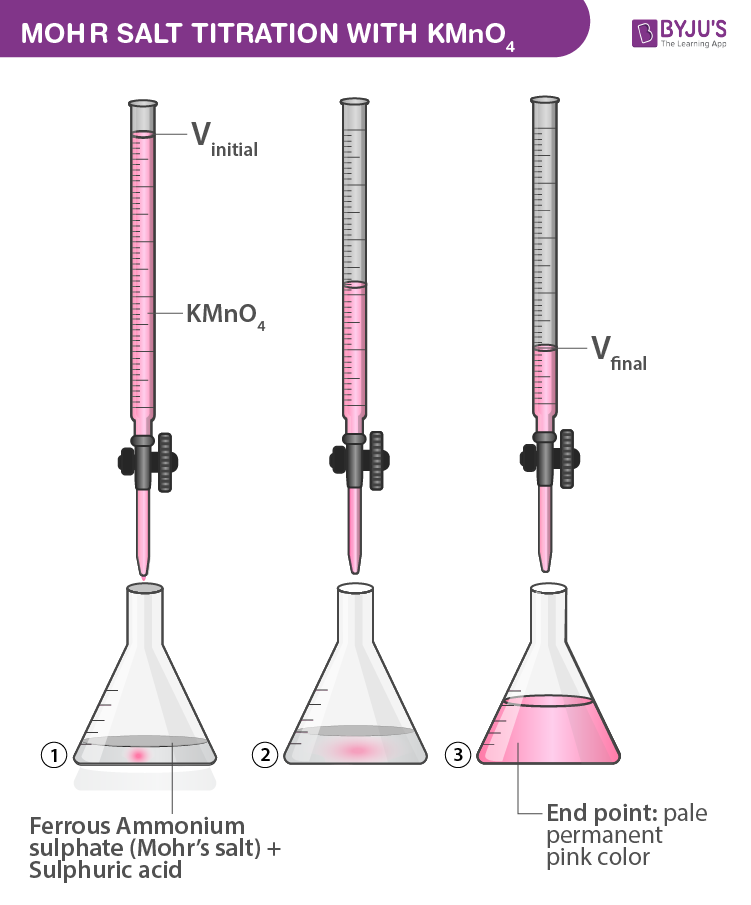
- In burette – KMnO4 solution
- In Conical flask – 10ml of Ferrous Ammonium Sulfate (Mohr’s salt) + Sulfuric acid
- Indicator – Self indicator (KMnO4)
- End Point – Colourless to permanent pale pink colour.
Procedure:
(a) Preparation of 0.05M standard solution of ferrous ammonium sulfate:
The quantity of Mohr’s salt required for the 250ml of the solution having a normality of 0.05N can be calculated as follows.
The molar mass of mohr’s salt = 392 g/mol
Strength = Normality x Equivalent weight
= (1/20) x 392 = 19.6 g/L
For preparing 250ml of N/20 Mohr’s salt solution, Mohr salt required
= (19.6/1000) x 250 = 4.9 gm
- Weigh an empty watch glass using a chemical balance.
- Weigh accurately 4.9gm of Mohr’s salt in a chemical balance.
- With the help of a funnel, transfer the Mohr’s salt into the measuring flask.
- Now wash the funnel with distilled water without removing the funnel from the flask.
- Make the solution up to the marked point with distilled water and make sure the Mohr’s salt is fully dissolved.
- This solution is 0.05N standard solution of Mohr’s salt.
(b) Titration of potassium permanganate solution against standard ferrous ammonium sulfate (Mohr’s salt) solution:
- Wash and rinse the burette and pipette with distilled water and then rinse with the corresponding solution to be filled in them.
- Rinse the burette with the potassium permanganate solution and fill the burette with potassium permanganate solution.
- Fix the burette in the burette stand and place the white tile below the burette in order to find the endpoint correctly.
- Rinse the pipette and conical flask with standard ferrous sulfate solution.
- Pipette out 10ml of 0.05N standard Mohr’s salt solution into the conical flask.
- Add a test tube full of sulfuric acid in order to prevent oxidation of manganese to form manganese dioxide.
- Note down the initial reading in the burette before starting the titration.
- Now start the titration, titrate against potassium permanganate solution and simultaneously swirl the solution in the flask gently.
- Initially, the purple colour of KMnO4 is discharged with ferrous ammonium sulfate. The appearance of a permanent pink colour reveals the endpoint.
- Repeat the titration until concordant values are obtained.
- Note down the upper meniscus on the burette readings.
- Record the reading in the observation table given below in order to calculate the molarity of KMnO4 given.
Observations:
| S.No | Volume of ferrous ammonium sulfate (Mohr’s salt) used | Burette Reading | Volume(V) of KMnO4 used
V = (y-x)ml |
|
| Initial(x) | Final(y) | |||
Calculations:
(a) Normality of KMnO4 solution:
Consider y ml of given KMnO4 solution is equivalent to 20ml of N/10 Mohr’s salt solution.
According to law of equivalents,
N1V1 = N2V2
- N1, N2 are normality of Mohr’s salt and KMnO4 solution respectively.
- V1, V2 are volumes of Mohr’s salt and KMnO4 respectively.
1/10 x 20 = N2 x y
N2 = 2/y
N = Normality of given KMnO4 solution = 2/y
(b) Strength of KMnO4 solution:
Strength = Normality x Equivalent mass
Equivalent mass of KMnO4 =
= 158/5
= 31.6
= 2/y x 31.6 g/liter
Molarity of KMnO4 solution
N = M x Number of electron gained
N = M x 5
M = N/5 moles/ litre
The strength of and molarity of given KMnO4 solution is found out as 2/y x 31.6 g/l and N/5 moles/liter, respectively.
Results and Discussion:
- Molarity of given KMnO4 solution is _________ moles/liter
- The strength of given potassium permanganate solution is _______ g/L
Precautions:
- Potassium permanganate is dark, so always read the upper meniscus.
- Rinse the pipette and burette before use.
- Use dilute sulfuric acid for acidifying the potassium permanganate.
- Clean all the apparatus with distilled water before starting the experiment and then rinse with the solution to be taken in them.
- Take accurate readings once it reaches the endpoint and doesn’t go with average readings.
- Do not use a rubber cork burette as it can be attacked by KMnO4.
- Use the antiparallel card or auto parallax card while taking the burette readings.
- The strength of the unknown solution should be taken up to two decimal places only.
| Also Read: |
Viva Questions on Mohr Salt Titration with KMnO4
Define titrant and titre.
The titre of a solution is the weight of a substance that reacts with 1 mL of the solution, such as a titrant.
Why is dil.sulfuric acid suitable for permanganate titration?
KMnO4 acts as a good oxidising agent in acidic medium. If acid is not used KMnO4 may be oxidised to MnO2 giving a brown precipitate.
What is the formula for Mohr’s salt?
The formula for Mohr’s salt is (NH4)2Fe(SO4)2.6H2O.
What is the standard solution?
A standard solution is a solution whose concentration is known. The normality and molarity of the solution is known.
What are the different types of titration?
The different types of titration are:
- Iodometric titration
- Permanganate titration
- Complexometric titration
- Precipitation titration
- Acid-base titration
- Redox titration
Recommended Videos
Titration using acidified KMnO4 and acidified K2Cr2O7
Keep visiting BYJU’S to learn more about Class 12 CBSE chemistry practicals.


Comments