What are the Properties of Alcohols?
The physical and chemical properties of alcohols are mainly due to the presence of hydroxyl group.
Alcohols are organic compounds in which a hydrogen atom of an aliphatic carbon is replaced with a hydroxyl group. Thus, an alcohol molecule consists of two parts; one containing the alkyl group and the other containing functional group hydroxyl group. They have a sweet odour. They exhibit a unique set of physical and chemical properties.
Table of Contents
- Recommended Videos
- Physical Properties of Alcohol
- Chemical Properties of Alcohols
- Frequently Asked Questions – FAQs
Recommended Videos

Some prominent physical and chemical properties of alcohols are given below.
Physical Properties of Alcohol
1. The Boiling Point of Alcohols
Alcohols generally have higher boiling points in comparison to other hydrocarbons having equal molecular masses. This is due to the presence of intermolecular hydrogen bonding between hydroxyl groups of alcohol molecules. In general, the boiling point of alcohols increases with an increase in the number of carbon atoms in the aliphatic carbon chain. On the other hand, the boiling point decreases with an increase in branching in aliphatic carbon chains, the Van der Waals force decreases with a decrease in surface area. Thus primary alcohols have a higher boiling point.
2. Solubility of Alcohols
The solubility of alcohol in water is governed by the hydroxyl group present. The hydroxyl group in alcohol is involved in the formation of intermolecular hydrogen bonding. Thus, hydrogen bonds are formed between water and alcohol molecules which make alcohol soluble in water. However, the alkyl group attached to the hydroxyl group is hydrophobic in nature. Thus, the solubility of alcohol decreases with the increase in the size of the alkyl group.
3. The Acidity of Alcohols
Alcohols react with active metals such as sodium, potassium etc. to form the corresponding alkoxide. These reactions of alcohols indicate their acidic nature. The acidic nature of alcohol is due to the polarity of –OH bond. The acidity of alcohols decreases when an electron-donating group is attached to the hydroxyl group as it increases the electron density on the oxygen atom. Thus, primary alcohols are generally more acidic than secondary and tertiary alcohols. Due to the presence of unshared electrons on the oxygen atom, alcohols act as Bronsted bases too.
Chemical Properties of Alcohols
Alcohols exhibit a wide range of spontaneous chemical reactions due to the cleavage of the C-O bond and O-H bond. Some prominent chemical reactions of alcohols are:
1. Oxidation of Alcohol
- Alcohols undergo oxidation in the presence of an oxidizing agent to produce aldehydes and ketones which upon further oxidation give carboxylic acids.
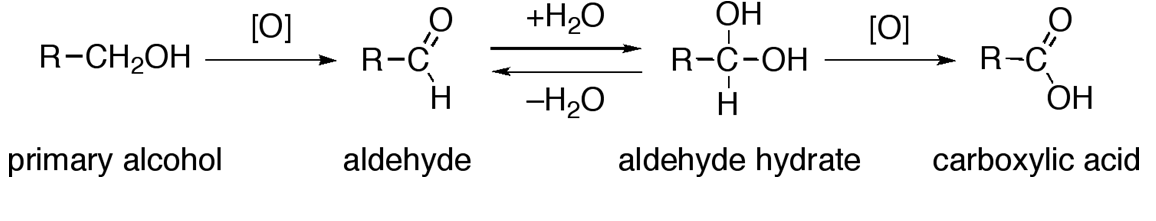
Alcohols: Physical and Chemical Properties
2. Dehydration of Alcohol
- Upon treatment with protic acids, alcohols undergo dehydration (removal of a molecule of water) to form alkenes. Dehydration of alcohol
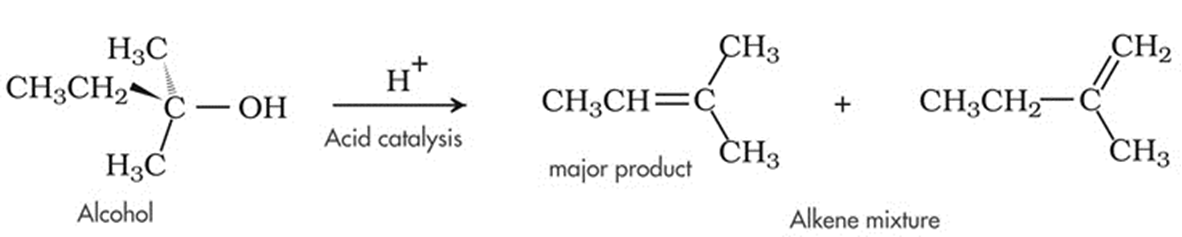
3. Catalytic Reduction of Butanal
Reduction of butanal produces butanol. This is occurs by hydrogenation reaction. Here the hydrogens are added to the carbon – oxygen double bond, it is converted to a carbon – oxygen single bond, as the carboxyl oxygen becomes a hydroxyl group.
Addition of hydrogen to a carbon-carbon double bond to form an alkane is a reduction reaction that is also called catalytic hydrogenation. Hydrogenation of a double bond is a thermodynamically favourable reaction because it forms a more stable (lower energy) product.
Frequently Asked Questions – FAQs
What are the physical and chemical properties of alcohols and phenols?
Lower alcohols are colourless liquids at normal temperature. The higher alcohols are colourless, odourless waxy solids. Phenols, like alcohols, are either colourless liquids or solids. But they usually turn reddish brown due to atmospheric oxidation.
What is the physical properties of alcohol?
Most of the common alcohols are colourless liquids at room temperature. Methyl alcohol, ethyl alcohol, and isopropyl alcohol are free-flowing liquids with fruity odours. The higher alcohols—those containing 4 to 10 carbon atoms—are somewhat viscous, or oily, and they have heavier fruity odours.
What are 2 chemical properties of alcohol?
Alcohols are acidic in nature. They react with metals such as sodium, potassium etc. It is due to the polarity of bond between hydrogen atom and oxygen atom of hydroxyl group. Primary alcohols are more acidic than secondary and tertiary alcohols.
What are the properties and uses of alcohol?
Isopropyl alcohol is widely used in industry as a solvent for paints and chemical processes. In addition to its presence in alcoholic beverages, ethanol also is used as a solvent for food extracts such as vanilla, perfumes, and some types of paints and lacquers.
What are the properties of phenols?
Phenols are similar to alcohols but form stronger hydrogen bonds. Thus, they are more soluble in water than alcohols and have higher boiling points. Phenols occur either as colourless liquids or white solids at room temperature and may be highly toxic and caustic.
For detailed discussions on physical and chemical properties of alcohols, download BYJU’S- The Learning App.

Great at giving more information to increase the understanding of a certain subject
how much is isopropyl alcohol volatile? is it completely evaporate at normal room temperature?
Yes, isopropyl alcohol can undergo evaporation at room temperature. However, the boiling point of this compound is 82.6 degrees celsius.