What is Sodium Chloride?
Sodium chloride is an ionic compound in which the sodium and chloride ions are in the ratio of 1:1. It is commonly called table salt, common salt or halite (the mineral form of common salt).
It is the salt which is mainly responsible for the salinity of seawater and for the extracellular fluid which is present in many multi-cellular organisms. It finds its application from household to industrial processes. Seawater is a major source of this salt.
Table of Contents
- The Occurrence of Sodium Chloride
- Preparation of Sodium Chloride
- Properties of Sodium Chloride
- Uses of Sodium Chloride
- Recommended Videos
- Frequently Asked Questions – FAQs
The chemical formula of sodium chloride is NaCl.
The Occurrence of Sodium Chloride
Mostly all the chemical compounds which consist of chlorine or sodium are usually derived from salts. It is distributed abundantly in nature. Salt is a major ingredient of the dissolved materials in seawater.
Pure salt can be obtained from mineral halite. Sodium chloride is obtained by mining the deposits and brine solution is obtained by passing water into the deposits. Hence the salts get dissolved then the solution is pumped out.
Evaporation of the seawater is one of the major processes used to obtain salt and is most widely followed in countries like India. The crystals obtained usually consist of impurities such as calcium sulfate, sodium sulfate etc. Pure crystals are obtained by dissolving the salts with little water and filtering the solution.
Preparation of Sodium Chloride
However, sodium and chlorine respond together to generate a substance that is familiar to nearly everybody around the globe that is sodium chloride, table salt, or common salt.
2Na(s) + Cl2(g) → 2NaCl(s)
Properties of Sodium Chloride
- It is easily soluble in water and partially soluble or insoluble in other liquids.
- They are white crystals which do not have an odour but possess a taste.
- In its aqueous state NaCl acts as a good conductor of electricity due to the free movement of the ions.
- It has a melting point of 801°C and a boiling point of 1,413°C.
Uses of Sodium Chloride
- It is widely used in food industries as a food preservative and as a flavour enhancer.
- It is a major raw material in the industrial manufacturing of various chemicals such as sodium carbonate, sodium hydrogen carbonate etc.
- This salt is used in glass production.
- In cold countries, it is used to prevent the build-up of ice on roads, bridges etc which is important for safe driving conditions.
Recommended Videos

Frequently Asked Questions – FAQs
What is sodium chloride used for?
The basic compound used by our body to digest and transport nutrients is sodium chloride ( NaCl), also known as salt. Preservation of blood pressure. Keeping the correct fluid balance.
What are the uses of saline solution?
Saline solution is commonly referred to as regular saline, although it is sometimes referred to as isotonic or physiological saline. In medicine, saline has many applications. It’s used for wound washing, sinus clearance, and dehydration care. It may be topically added or intravenously used.
Why the formula of sodium chloride is NaCl?
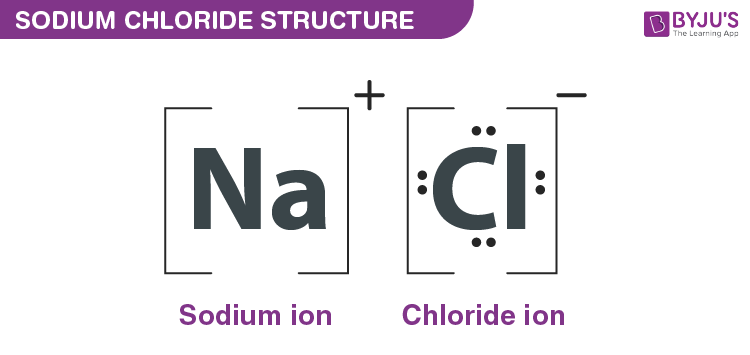
If sodium atoms interact with chlorine atoms, sodium chloride is formed. Sodium will donate an electron (which is a negative-charged particle) to chlorine as this happens. The chemical formula for sodium chloride is NaCl, indicating that there is precisely one chloride atom for every sodium atom present.
Does sodium chloride kill bacteria?
Sodium chloride is not only used for a number of different thing, but is a good antibacterial agent as well. An antibacterial agent is one that prevents bacteria from developing and multiplying.
What is the primary composition of NaCl?
Formula and structure: NaCl is the molecular formula of sodium chloride and 58.44 g / mol is its molar mass. It is an ionic compound which consists of a chloride anion (Cl-) and a sodium cation (Na+).
Register with BYJU’S to learn more on the topic and several other topics of chemistry.


Thank you so much.