What are Saturated Hydrocarbons?
A Saturated hydrocarbon is a hydrocarbon in which all the carbon-carbon bonds are single bonds. A hydrocarbon is an organic compound whose only constituents are carbon and hydrogen. As the name suggests, saturated hydrocarbons are hydrocarbons in which all the carbon atoms are bonded to four other atoms and are ‘saturated’, implying that no carbon-carbon multiple bonds exist in these organic compounds.
Table of Contents
Generally, the term ‘saturated hydrocarbon’ is used to refer to alkanes – acyclic hydrocarbons containing only sp3 hybridized carbon atoms. The general formula of an alkane is CnH2n+2. An illustration describing the structure of a propane molecule (C3H8) is provided below.
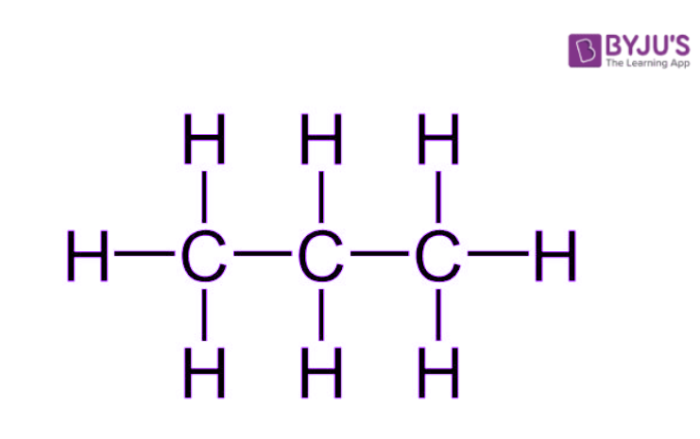
It can be observed that a propane molecule is ‘saturated’ with carbon-carbon single bonds and the rest of the valencies of carbon are satisfied by hydrogen atoms A few other examples of saturated hydrocarbons are listed below.
-
-
-
- Butane (C4H10)
- Octane (C8H18)
- Cyclohexane (C6H12)
- Cyclopropane (C3H6)
-
-
Cycloalkanes are alkanes that have a monocyclic ring structure. Since all the carbon-carbon bonds in cycloalkanes are single bonds, they are considered to be saturated hydrocarbons. Therefore, the general formula of a saturated hydrocarbon can be written as CnH(2n + 2 – 2r), where ‘r’ is the total number of rings in the molecule.
Recommended Videos

Difference Between Saturated and Unsaturated Hydrocarbons
Unsaturated hydrocarbons feature at least one double or triple bond between two adjacent carbon atoms. The key differences between saturated and unsaturated hydrocarbons are tabulated below.
| Saturated Hydrocarbons | Unsaturated Hydrocarbons |
| All carbon atoms are sp3 hybridized in these compounds. | They contain sp2 or sp hybridized carbons. |
| Contain more hydrogen atoms than the corresponding unsaturated hydrocarbons. | Contain fewer hydrogens than the corresponding saturated hydrocarbons. |
| Examples include alkanes and cycloalkanes. | Examples include alkenes, alkynes, and aromatic hydrocarbons. |
| They have a relatively low chemical reactivity | They are more reactive than their saturated counterparts. |
| They generally burn with a blue flame | These generally burn with a sooty flame. |
Types of Saturated Hydrocarbons
A saturated hydrocarbon can have a linear, branched, or ring-shaped structure and can, therefore, be classified into one of the following types:
-
-
-
- Alkanes
- Cycloalkanes
-
-
It is important to note that even polycyclic alkanes (alkanes with several rings in their structures) are also categorized as cycloalkanes and are, therefore, a type of saturated hydrocarbons.
Alkanes
Alkanes can feature a linear or a branched carbon chain in their structures. All the carbon atoms in these saturated hydrocarbons are sp3 hybridized. The melting point and the boiling point of an alkane are related to the length of its carbon chain. The longer the chain, the higher the melting or boiling point. This is because molecules having long carbon chains have high molecular weights.
At standard temperatures, alkanes containing up to four carbon atoms are gases, and those containing 5 to 17 carbon atoms are liquids. Alkanes that have more than 18 carbon atoms are solids at room temperature.
All alkanes containing more than 3 carbon atoms can exhibit chain isomerism. Chain isomers have the same molecular formula but a different number of carbon atoms in their parent chain. An illustration describing the possible chain isomers of butane (C4H10) is provided below.
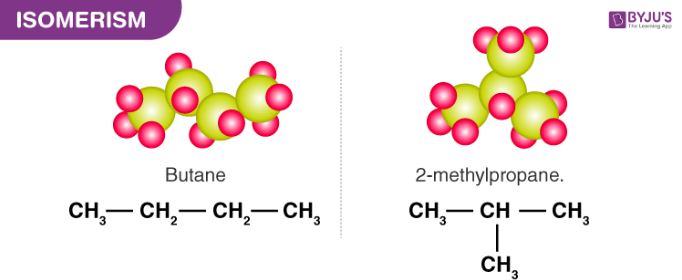
The number of possible chain isomers for an alkane depends on the total number of carbon atoms in it. For example, butane has only 2 chain isomers but octane (C8H18) has a total of 18 possible chain isomers.
Cycloalkanes
-
-
-
- Cycloalkanes feature a ring-shaped arrangement of sp3 hybridized carbon atoms. However, the ring in these saturated hydrocarbons can branch into side chains.
- The physical properties of cycloalkanes are somewhat similar to those of alkanes.
- The melting and boiling points of cycloalkanes are generally higher than the alkanes containing the same number of carbon atoms.
- Owing to their structures, cycloalkanes are subject to ring strain. Since cyclopropane has a carbon-carbon bond angle of 60o, it has the highest ring strain among all cycloalkanes.
-
-
What are the Uses of Saturated Hydrocarbons?
Alkanes are widely used as fuels, heating oils, and solvents. A few other uses of saturated hydrocarbons are listed below.
-
-
-
- Methane is used as a fuel in several automobiles, water heaters, and ovens. In its refined form, liquid methane can also serve as rocket fuel.
- Several cryogenic refrigeration systems use ethane as a refrigerant. It is also used in the production of ethylene.
- The propellant used in several aerosol sprays is a saturated hydrocarbon known as propane. This compound is also used as a fuel for hot air balloons.
- Octane is a very important component of gasoline since it helps prevent engine damage.
- Cycloalkanes also find use in motor fuels, diesel, petroleum gas, and other heavy oils.
- The manufacture of rubber and nylon also involves the use of cycloalkanes.
-
-
Frequently Asked Questions – FAQs
What are saturated hydrocarbons?
What type of isomerism is shown by alkanes?
Among alkanes and alkenes, which are more reactive and why?
Why are cycloalkanes less stable?
What are some uses of alkanes?
To learn more about saturated hydrocarbons and other related concepts in organic chemistry (such as the combustion of hydrocarbons), register with BYJU’S and download the mobile application on your smartphone.

i really enjoy this site