What is Wilkinson’s Catalyst?
- Wilkinson’s Catalyst [IUPAC Name: chloridotris(triphenylphosphine)rhodium(I)] is a coordination compound whose coordination centre is rhodium.
- It is extensively used as a catalyst in the hydrogenation of alkenes.
- The chemical formula of Wilkinson’s catalyst can be written as [RhCl(PPh3)3] where ‘Ph’ denotes a phenyl group.
- At ambient temperatures, this coordination complex exists as a reddish-brown solid.
Wilkinson’s catalyst is named after the English chemist Sir Geoffrey Wilkinson. Its structure is illustrated below.

It can be noted that as per single-crystal X-ray diffraction studies, the structure adopted by Wilkinson’s Catalyst is square planar (slightly distorted). The rhodium centre is bound to four ligands in this compound.
Table of Contents
- Properties of Wilkinson’s Catalyst
- Preparation of Wilkinson’s Catalyst
- Mechanism of Catalysis for the Hydrogenation of Alkenes
- Applications of Wilkinson’s Catalyst
Properties of Wilkinson’s Catalyst
Physical Properties
- The molar mass of Wilkinson’s catalyst is 925.22 grams/mole
- It’s melting point ranges from 518 to 523K
- It is insoluble in water. However, it is soluble in many hydrocarbon-based solvents such as benzene and tetrahydrofuran
Chemical Properties
- Wilkinson’s catalyst has a square planar coordination geometry
- It reacts with carbon monoxide to yield [RhCl(CO)(PPh3)2]
- This coordination compound undergoes dimerization when stirred into a solution of benzene. The chemical composition of the dimer can be expressed as [RhCl(PPh3)2]2
Preparation of Wilkinson’s Catalyst
Wilkinson’s catalyst can be prepared by reacting hydrated rhodium(III) chloride with excess triphenylphosphine in the presence of ethanol (which acts as a refluxing agent). Here, the triphenylphosphine (denoted by the chemical formula P(C6H5)3) acts as a reducing agent which has the ability to oxidise itself from an oxidation state of +3 to an oxidation state of +5.
During the synthesis of Wilkinson’s catalyst, one equivalent of triphenylphosphine reduces rhodium(III) to rhodium(I) while three other equivalents bind themselves to the metal as ligands in the final product.
4P(C6H5)3 + RhCl3(H2O)3 → RhCl(P(C6H5)3)3 + OP(C6H5)3 + 2HCl + 2H2O
The chemical reaction for the synthesis of Wilkinson’s catalyst is provided above.
Mechanism of Catalysis for the Hydrogenation of Alkenes
- Initially, a 14-electron or 12-electron complex is formed from the dissociation of 1 or 2 triphenylphosphine ligands.
- Now, the oxidative addition of molecular hydrogen (H2) to the metal core of Wilkinson’s catalyst (rhodium) occurs.
- The third step of the mechanism involves the formation of a pi complex with the alkene.
- The hydrogen is inserted into the complex via migratory insertion which could proceed through intramolecular hydride transfer or through olefin insertion.
- Finally, reductive elimination occurs at the pi complex to regenerate the catalyst and afford the required alkene product.
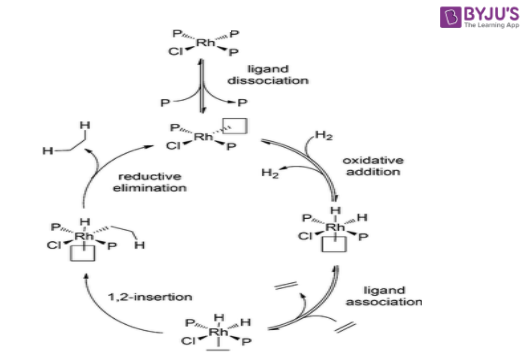
An illustration detailing the mechanism of action of Wilkinson’s catalyst for the hydrogenation of olefins is provided above.
Applications of Wilkinson’s Catalyst
- Wilkinson’s catalyst can be employed for the hydroacylation of alkenes.
- The hydroboration and hydrosilylation of olefins can also be achieved with the help of this coordination complex.
- Functionalized tri-substituted alkenes and internal alkynes can be subjected to hydrogenation with the help of Wilkinson’s catalyst in the presence of hydrogen and a strong base. Here, a highly reactive Rh(I) species having relatively superior catalytic activity is formed
- This catalyst is highly effective in the selective reduction of the least hindered olefin when there are several olefins present.
To learn more about Wilkinson’s catalyst and other important catalysts such as Lindlar catalysts, register with BYJU’S and download the mobile application on your smartphone.

Comments