Emulsions are the mixtures of two or more types of liquids where, one is such as droplets of tiny or even ultramicroscopic size, which are distributed throughout each other. These are usually formed from the component of liquids either in natural form or, more often, using mechanisms, such as agitation, provided that these fluids mixed have no kind of mutual solubility.
Download Complete Chapter Notes of Surface Chemistry
Download Now
Emulsions are said to be stabilised by some agents, forming films at the surface of droplets or those which impart to them a kind of mechanical stability. The unstable form of emulsions eventually separates into two forms of liquid layers. The stable emulsions are destroyed by deactivating the emulsifying agent, for example, by the addition of appropriate third-party substances or even by the process of freezing or heating.
Some common emulsions are milk (where the dispersion of fat molecules or droplets in the aqueous solution) and also butter (which is the dispersion of droplets of particles of an aqueous solution in the fat).
Table of Contents
- What Is Emulsion?
- Emulsion Examples
- Properties of Emulsion
- Types of Emulsions
- Emulsifier/Emulsifying Agent
- How Do Emulsifiers Work?
- Identifying Types of Emulsions
- Applications and Uses of Emulsions
What Is Emulsion?
An emulsion can be defined as a colloid consisting of two or more non-homogenous types of liquids wherein one of the liquids contains the dispersion of the different forms of liquids.
Emulsion Examples
Emulsions basically consist of a dispersion of two liquids that are immiscible with each other. One of the liquids will act as the dispersion medium, and the other will act as the dispersed phase. In simple words, emulsions are colloids in which both the dispersed phase and dispersion medium are liquids. Oil and the mixtures of water are emulsions when shaken together. The oil forms a drop and then disperses throughout the water.
The term emulsion is also applied to a group of mixed systems called solutions or gels, or suspensions. Take, for example, the photographic emulsion is a gelatin gel consisting of tiny crystals dispersed in it. Some other examples of emulsions include butter which is an emulsion of water in fat, and egg yolk containing lecithin.
| DISPERSED PHASE | DISPERSION MEDIUM | TYPE OF COLLOID | EXAMPLE |
| Solid | Solid | Solid | Some Coloured Glasses and Gemstones |
| Solid | Liquid | Solid | Paints, Cell Fluids |
| Solid | Gas | Aerosol | Smoke, Dust |
| Liquid | Solid | Gel | Cheese, Butter, Jellies |
| Liquid | Liquid | Emulsion | Milk, Hair Cream |
| Liquid | Gas | Aerosol | Fog, Mist, Cloud, Insecticide Sprays |
| Gas | Solid | Solid | Pumice Stone, Foam Rubber |
| Gas | Liquid | Foam | Froth, Whipped Cream, Soap Lather |
Properties of Emulsions
- Emulsions are both continuous and dispersed, with the boundary coming between the phases that are called “interface”.
- Emulsions have a cloudy appearance due to many phase interfaces scattering light passing through the emulsions.
- Emulsions appear in white colour when the light is dispersed in equal proportions.
- If the emulsion is dilute, then higher-frequency and low-wavelength types of light will be scattered in more fractions, and this kind of emulsion will appear in blue colour. This is also referred to as the Tyndall effect.
Also Read: Tyndall Effect and Dispersion of Light
Types of Emulsions
Emulsions can be classified on the basis of the properties of the dispersed phase and the dispersion medium.
1) Oil in water (O/W):
In this type of emulsion, the oil will be the dispersed phase, and water will be the dispersion medium. The best example of o/w emulsion is milk. In milk, the fat globules (which act as the dispersed phase) are suspended in water (which acts as the dispersion medium).
2) Water in oil (w/o):
In this type, water will be the dispersed phase, and oil will be the dispersion medium. Margarine (a spread used for flavouring, baking and working) is an example of water in oil emulsion.
Emulsifier/Emulsifying Agent
These are substances which are added to emulsions for stabilisation purposes. The various characteristics of emulsifiers are given below:
- They are substances which have a hydrophilic end (polar) as well as a hydrophobic end (non-polar).
- They are soluble in both water and oil.
- Emulsifiers form a layer between the dispersed phase and the dispersion medium, thereby preventing the dispersed phase particles from coming together to form larger particles and separate out.
- Emulsifiers can be cationic, anionic or even non-polar.
- It is not just the percentage of water and oil which decides whether it is an oil-in-water or a water-in-oil emulsion. On the other hand, it depends on which water and oil can solvate the emulsifier to a larger extent.
- If the emulsifier is more soluble in water, then the water becomes the dispersion medium, and oil becomes the dispersed phase, and hence we get oil in water emulsion.
- On the other hand, if the emulsifier is more soluble in oil, then oil becomes the dispersion medium, and water becomes the dispersed phase.
- The commonly used emulsifiers for o/w emulsions are proteins, gums, soaps, etc.
- The commonly used emulsifiers for w/o emulsions are heavy metal salts of fatty acids, long-chain alcohols, etc.
Some common examples of emulsifiers are egg yolk, mustard, sodium phosphates, DATEM, mono- and diglycerides, cellulose, soy lecithin, etc.
How Do Emulsifiers Work?
To understand this, firstly, we need to understand the process of coalescing. Coalescing is the process in which similar particles in the emulsions come together to form larger and bulkier particles, leading to the separation of the dispersed phase and dispersion medium.
Emulsifiers help in preventing coalescing by forming a physical barrier between the dispersed phase and the dispersion medium. As we have seen before, emulsifiers, like soap, have both a hydrophilic end and a hydrophobic end. Hence, they can attach to both polar and non-polar substances. Let us take the example of sodium stearate. C17H35COO–Na can be represented as,
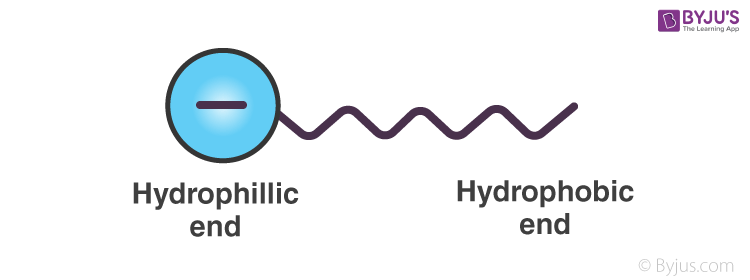
When this is added to an o/w emulsion, molecules of C17H35COO– surround the oil droplet with their non-polar tails/hydrophobic end (the hydrocarbon chain), extending into the oil and their polar heads/hydrophilic end (the carboxylate ion), facing the water as given in the figure.
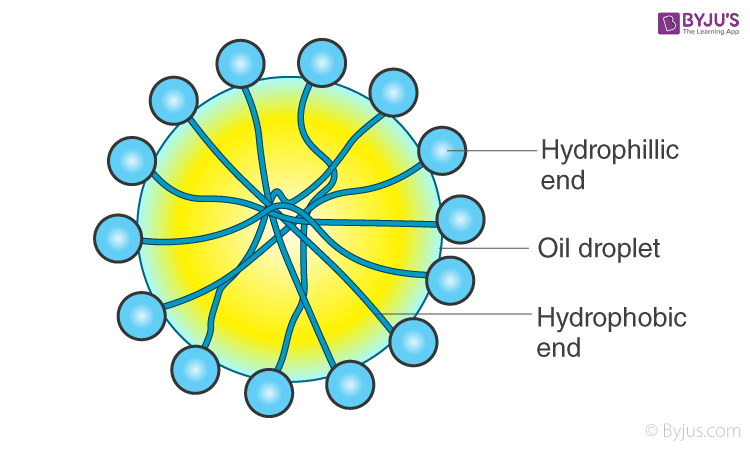
This arrangement brings a stronger adhesive force between the oil (dispersed phase) and water (dispersion medium). This newly formed adhesive force will be more than the cohesive force between oil-oil and water-water. Hence, oil particles will not have the tendency to come together to form larger particles. This helps in preventing coalescence, thereby stabilising the emulsion.
Note:
For w/o emulsion, the orientation of the emulsifier would be the opposite as that of o/w, i.e., non-polar (hydrophobic end) tail extends outside, and the polar (hydrophilic end) head faces inwards.
Theories of Emulsification
Since there are different processes and mechanisms (both chemical and physical) involved in the process of emulsification, there are several theories that accompany it.
Surface Tension Theory: This theory states or describes emulsification as a process that occurs by the reduction of interfacial tension between two phases.
Repulsion Theory: With this theory, we learn that the emulsifying agent produces a film over one phase, which further leads to the formation of globules. These compounds tend to repel each other, and the repulsive force that exists between them helps them to remain suspended in the dispersion medium.
Also Read: Thin Film Interference
Methods to Identify the Type of Emulsions
1) Dilution test
On adding water to an o/w emulsion, it will still remain stable as water is the dispersion medium, but on adding oil, it will get destabilised, as oil and water are immiscible. Similarly, w/o emulsion can be diluted with oil and would still be stable but would get destabilised by the addition of water.
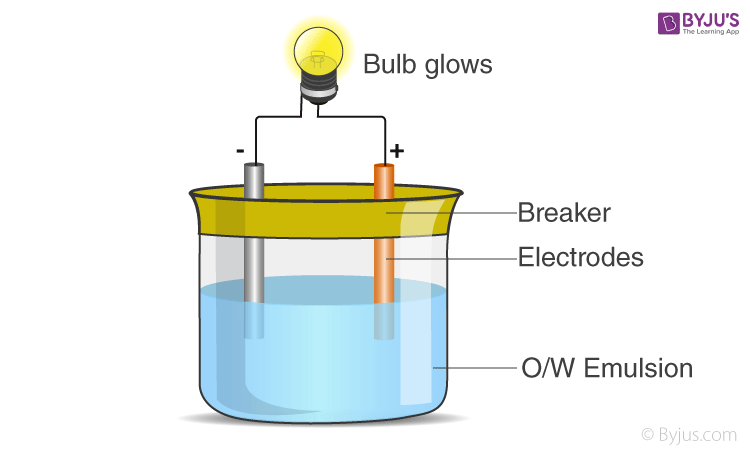
2) Conductivity test
In this test, the emulsion is kept between 2 electrodes and a bulb is connected to the circuit, as shown in the diagram. An o/w emulsion will conduct electricity as water conducts electricity, but a w/o will not conduct electricity.
3) Dye test
In this, a water-soluble dye is added to the emulsion. If it is an o/w emulsion, the dispersion medium appears red, and the dispersed phase is colourless and vice-versa.
Separation of Emulsions
The different methods by which emulsions can be separated into their constituent liquids include the following:
1) Heating
2) Centrifuging
Applications and Uses of Emulsion
Emulsions are very well-known in various fields of science. It is used in the tanning and dyeing industries, in the manufacturing process of plastics and synthetic rubber, etc.
- Usually used in cosmetics, pharmaceuticals, and personal hygiene.
- Microemulsions are used to deliver vaccines to kill various microbes.
- It is used in chemical synthesis, mainly in the manufacture of polymer dispersions.
- It is used in firefighting.
- Nanoemulsions such as soybean oil are used to kill microbes.
- Mayonnaise is an oil-in-water emulsion with egg yolk or sodium stearoyl lactylate.
Frequently Asked Questions on Emulsion
Are emulsions homogeneous?
Give an example of an emulsion.
What are the two types of emulsions?
1. Water in oil emulsion
2. Oil in water emulsion

Comments