What Is Kolbe’s Reaction?
Kolbe’s reaction, also known as Kolbe Schmitt Reaction, is a type of addition reaction named after Hermann Kolbe and Rudolf Schmitt. When phenol is treated with sodium hydroxide, phenoxide ion is generated. The phenoxide ion generated is more reactive than phenol towards electrophilic aromatic substitution reaction. Hence, it undergoes an electrophilic substitution reaction with carbon dioxide, which is a weak electrophile. Ortho-hydroxybenzoic acid (salicylic acid) is formed as the primary product. This reaction is popularly known as Kolbe’s reaction.
Download Complete Chapter Notes of Alcohols, Phenols and Ethers
Download Now
⇒ Jump to:
An example of the Kolbe’s reaction (Kolbe Schmitt Reaction) is given below:
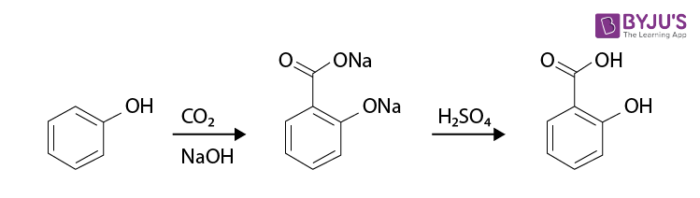
The mechanism of Kolbe’s reaction proceeds through the nucleophilic addition of phenoxide to carbon dioxide, yielding the salicylate.
The salicylate formed further reacts with the acid to form salicylic acid. It is a carboxylation reaction where sodium phenoxide is heated with carbon dioxide under a pressure of 100 atmospheres and a temperature of 125 degrees Celsius, and the resulting product is treated with sulfuric acid to yield salicylic acid (an aromatic hydroxy acid).
Applications of Kolbe’s Reaction
- When Potassium Hydroxide is used in Kolbe’s reaction, 4-Hydroxybenzoic acid can be accessed. This is an important precursor for parabens (parahydroxybenzoate or ester of para-hydroxy benzoic acid, used as a biocide in cosmetic products).
- Kolbe’s reaction can also be used for the industrial synthesis of 3-hydroxy-2-naphthoic acid, which is a common precursor to azo dyes and pigments.
- Salicylic acid can be used to make aspirin by reacting it with acetic anhydride. Aspirin is commonly used as a painkiller.
⇒ Check: Name Reactions in Organic Chemistry
Kolbe’s Reaction Mechanism
Kolbe’s reaction can be classified as a carboxylation chemical reaction. The reaction occurs when sodium phenoxide is allowed to absorb carbon dioxide, and the resulting product is heated at a temperature of a 125-degree Celsius and a pressure of over a hundred atmospheres. An unstable intermediate is now formed.
This unstable intermediate goes through a proton shift, leading to the formation of sodium salicylate. Now, this mixture is treated with sulfuric acid. The acidification of the mixture yields salicylic acid. The illustration for the Kolbe’s reaction mechanism is given below:
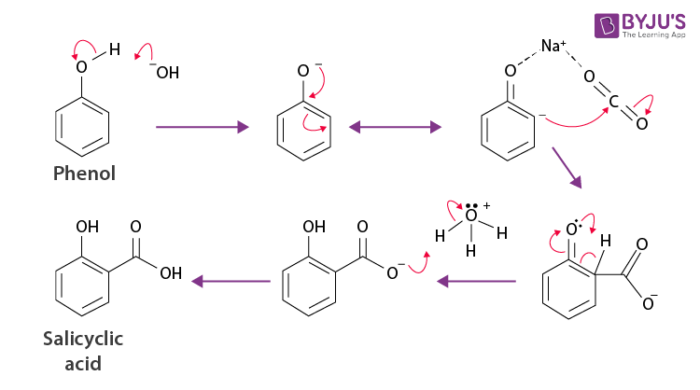
Thus, the required aromatic hydroxy acid – salicylic acid is produced via Kolbe’s reaction. It can be observed that there is a nucleophilic addition of sodium phenoxide to carbon dioxide gas to form the salicylate in the mechanism.
Frequently Asked Questions on Kolbe Reaction
Can Kolbe’s reaction be used for converting sodium acetate to ethane?
Yes, Kolbe’s reaction can be used in converting sodium acetate to ethane. Using the Kolbe electrolysis process, the aqueous solution of sodium acetate is electrolysed. The acetate ions get decomposed and form methyl radicals. These combine with other free methyl radicals, which leads to the generation of ethane.
Why do true aromatic acids reluctant to Kolbe’s electrolytic reaction?
The reason why true aromatic acids do not undergo Kolbe’s electrolytic reaction is that if we take aromatic carboxylic acid, the carboxyl group is attached to the benzene ring. This makes it difficult to lose CO2.
Why is sodium salicylate a major product in Kolbe’s reaction?
Kolbe’s reaction, in general, is a carboxylation chemical reaction which converts phenols to salicylate. However, we obtain sodium salicylate mainly due to the proton shift or the nucleophilic addition of a phenoxide, classically sodium phenoxide, to carbon dioxide.
Is Kolbe’s synthesis the same as Kolbe’s reaction or Kolbe’s electrolysis?
Yes, all the terms mean the same thing. The names are used interchangeably. Sometimes, Kolbe–Schmitt reaction or Kolbe’s process is also used.

Comments