What is Salicylic Acid?
Salicylic Acid is a type of beta hydroxy acid (BHA) and phenolic acid with a chemical formula C7H6O3. It is a BHA found as a natural compound in plants. It functions as a plant hormone. This lipophilic monohydroxybenzoic acid is a derivative of salicin metabolism. It is a crystalline organic carboxylic acid with keratolytic, bacteriostatic and fungicidal properties. It is poisonous when consumed in large. It can be used as an antiseptic and as a food preservative when consumed in small quantities. It consists of a carboxyl group COOH. It is odourless and has no colour.
Properties of Salicylic Acid – C7H6O3
| C7H6O3 | Salicylic Acid |
| Molecular Weight/ Molar Mass | 138.121 g/mol |
| Density | 1.44 g/cm³ |
| Boiling Point | 211 °C |
| Melting Point | 158.6 °C |
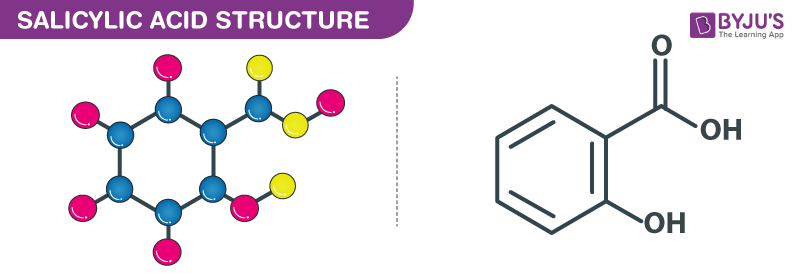
Salicylic Acid structure – C7H6O3
C7H6O3 Uses (Salicylic Acid)
- It is used in toothpaste as an antiseptic.
- In the medical field, it is used to remove the outer layer of the skin.
- It is used in the treatment of acne, dandruff, and wrath.
- It is used as a preservative.
- It is used in the production of drugs like aspirin.
- It is used as a balm to reduce muscle and joint pain.
- It is used to relieve pain caused by mouth ulcers.
- It is used as a key additive in skin care products.
Frequently Asked Questions
What are the uses of salicylic acid?
Salicylic acid acts as a keratolytic (it serves as a peeling agent). Salicylic acid facilitates and makes the outer layer of the skin shed. The topical salicylic acid (for the skin) is used to treat acne, dandruff, seborrhea, or psoriasis and to remove cotton, calluses, and warts. Salicylic acid is found in many daily-use products.
What does salicylic acid do to your skin?
Salicylic acid works by loosening and breaking apart desmosomes in the outer layers of the skin, which are attachments between cells. This action helps the skin to exfoliate and the pores to unclog. Salicylic acid is capable of reducing sebum secretion, which is another way to help reduce acne.
Is salicylic acid safe?
Although the use of low-concentration household salicylic acid products is usually considered safe, salicylic acid can cause mild chemical burns at high concentrations. These chemicals can also cause dangerous intoxication if ingested.
To learn more about the uses, properties and structure of salicylic acid (C7H6O3) from the experts register to BYJU’S now!


Comments