Markovnikov rule is one of the most important rules for the prediction of products of addition reaction of unsymmetrical alkenes in organic chemistry. Some FAQs related to Markovnikov rule are:
Important Questions For Markovnikov Rule
Question: What is the Markovnikov rule?
Answer: Markovnikov proposed a rule called Markovnikov rule for the prediction of major product in the electrophilic addition of unsymmetrical alkenes. According to Markovnikov rule, the negative part of the adding molecule gets attached to that carbon atom which possesses a lesser number of hydrogen atoms.
Question: What is Markovnikov addition reaction?
Answer: All the electrophilic addition reactions of alkenes following Markovnikov rule are known as Markovnikov addition reactions. A general example of such reaction is given below:
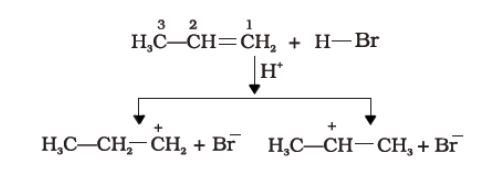
Questions: What is the general mechanism of electrophilic addition reaction following Markovnikov rule?
Answer: According to Markovnikov rule, addition reaction of alkenes follows the electrophilic addition reaction mechanism. The general mechanism is explained below:
- An electrophile, H+ is generated from hydrogen bromide which attacks the double bond to form a carbocation.
- Since the secondary carbocation is more stable than the primary carbocation, the secondary carbocation predominates the formation of ions.
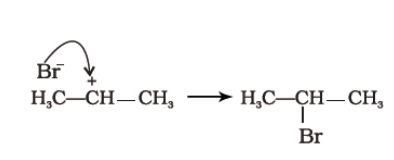
- Finally, Br– attacks the carbocation to form alkyl halides.
Questions: Which reference books can one follow for Markovnikov rule?
Answer: For understanding the Markovnikov rule, one must go through the NCERT class 11th chemistry textbook part-2. A brisk walk through hydrocarbons from NCERT will give you a sound idea of the addition reaction of unsymmetrical alkenes. Apart from this, you can follow a reference book to understand the rule in details. Some authentic books for understanding this rule include books from authors like Solomons & Fryhle, Morrison & Boyd. Once you have developed concepts on this topic, you can practice questions from the books of authors like M. S. Chauhan, etc.
For further details related to Markovnikov rule for JEE chemistry, get in touch with our mentors. To know more on How to study Chemistry for JEE and Important concepts for JEE Chemistry, keep visiting BYJU’S.
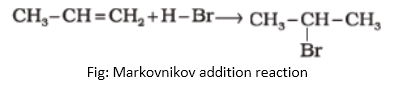
Comments