Students, before they learn about the hybridization of BrF5, should understand a few things about the chemical compound. BrF5 or Bromine Pentafluoride is a chemical compound that students might not come across frequently. It is a common chemical that is used mainly in rocket propellants and uranium processing. It is a colourless liquid and has properties like strong odour, high toxicity, and is corrosive in nature. This liquid reacts explosively when it comes in contact with organic materials.
The hybridization that takes place in BrF5 is sp3d2. We will look at how the process occurs below.
| Name of the Molecule | Bromine Pentafluoride |
| Molecular Formula | BrF5 |
| Hybridization Type | sp3d2 |
| Bond Angle | 90o |
| Geometry | Square Pyramidal |
What is the Hybridization of Bromine Pentafluoride?
If we look at the electron configuration of the Bromine atom it is represented as;
1s2 2s22p6 3s23p63d104s24p5. In order to obtain a pentavalency, some of the electrons are shifted to 4d-orbitals. Two of the p-orbitals also become unpaired. At this moment, the bromine atom will be in an excited state and hybridization occurs.
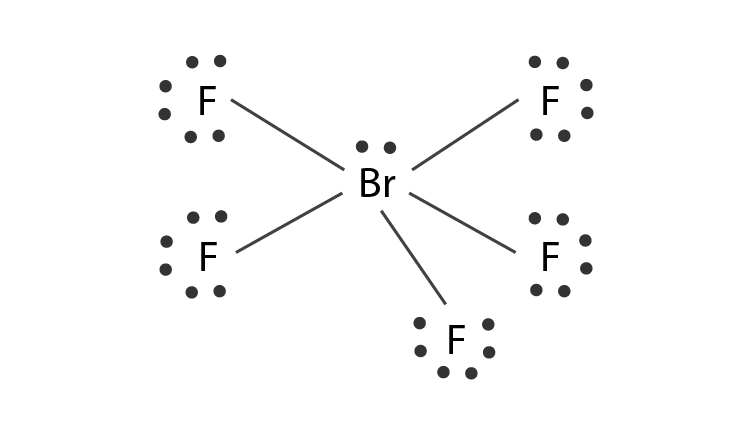
During the hybridization one 4s, three 4p and two 4d orbitals take part in the process giving rise to sp3d2 hybrid orbitals. Five valence electrons of bromine will be used to form sigma bonds with 5 F atoms. The molecule will consist of one lone pair.
Important Points To Remember
- In BrF5, one 4s, three 4p and two 4d orbitals take part in hybridization.
- The central atom bromine forms 5 sigma bonds with fluorine atoms.
- Lone pairs are found in one of the hybrid orbitals.
BrF5 Molecular Geometry And Bond Angles
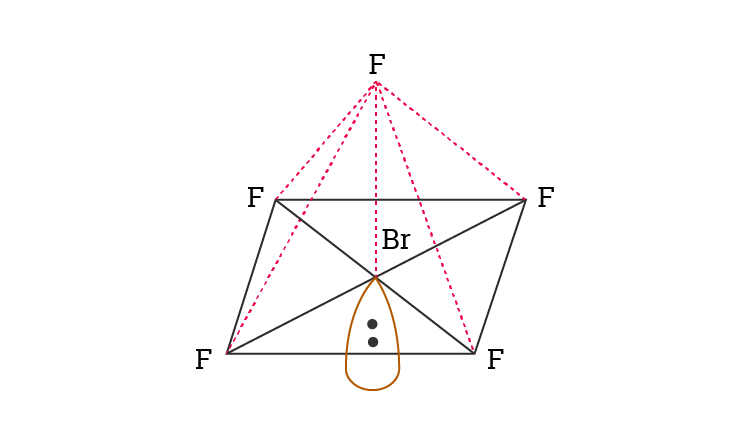
BrF5 molecular geometry is said to be square pyramidal with a bond angle of 90o each.
Read More About Hybridization of Other Chemical Compounds
- Hybridization Of XeF4
- Hybridization Of SF4
- Hybridization Of PCl3
- Hybridization Of Graphite
- Hybridization Of SO3

Comments